I. EFFECT OF Fetal Fraction (FF) on NIPT RESULTS
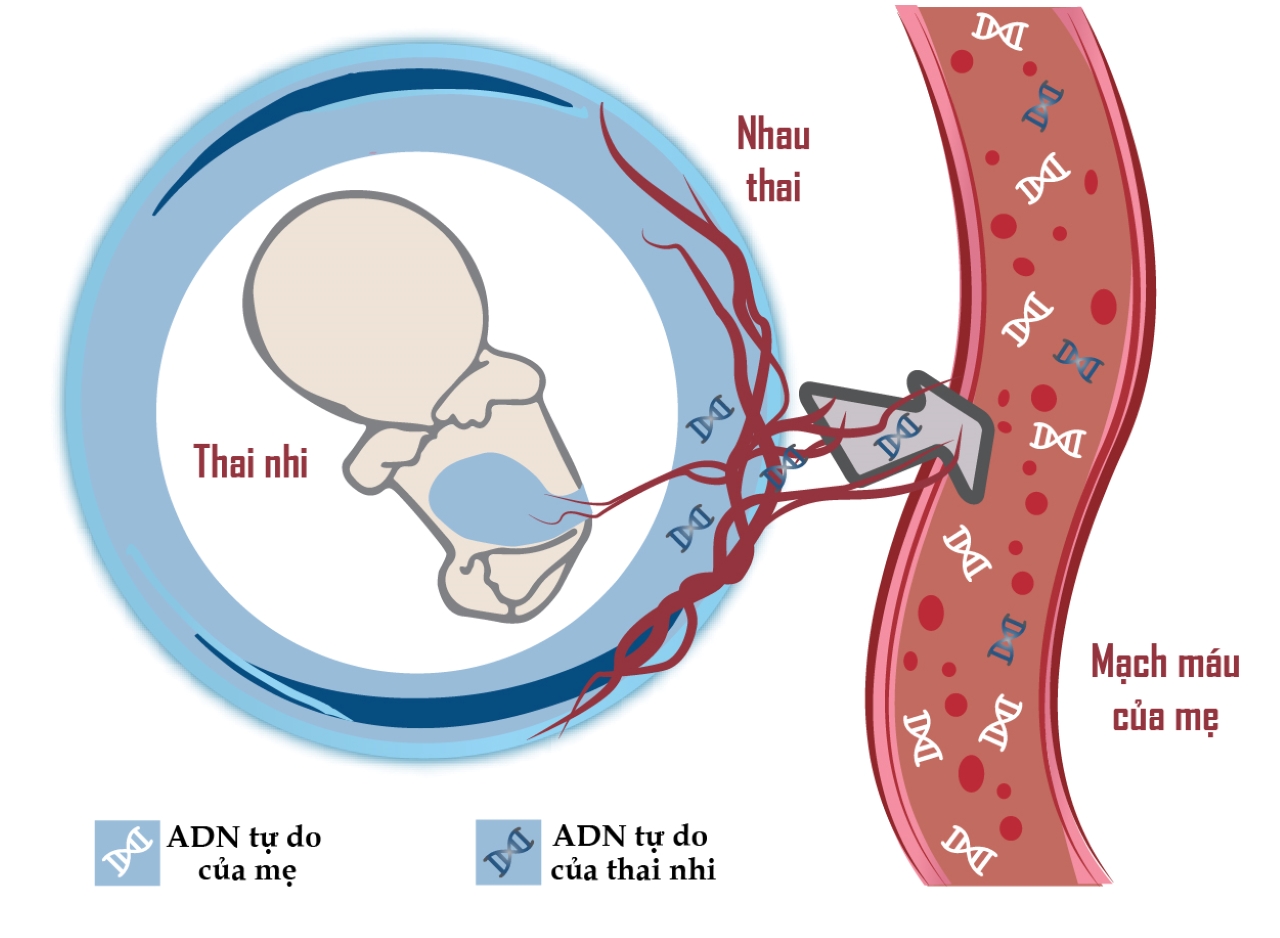
Non-invasive prenatal screening test (NIPT) is an advanced screening method to detect fetal aneuploidy through fetal free DNA (cfDNA) in maternal blood. Fetal cfDNA is derived from apoptosis of trophoblastic cells (syncytiotrophoblasts), while maternal hematopoietic cells are the source of most maternal cfDNA. Both the mother and the fetal placenta produce cfDNA and circulate in the maternal blood circulation, of which maternal cfDNA accounts for 90% (Figure 1).

In the NIPT test, the fetal fraction (FF) is considered an important factor and is the first factor to evaluate the quality of the test. FF is the ratio of fetal cfDNA to all circulating cfDNA in maternal plasma. As reported by Scheffer et al. 2021, the minimum FF to produce reliable results and minimize the number of false negatives as well as not giving results is 4%. However, for many modern methods used today, reliable results can be obtained at much lower FF levels (from 2.6% - 3.5%).
According to the 2020 meta-analysis data, between 1.7 and 8% of pregnant women with very limited amount of fetal DNA circulating in the blood have been reported in clinical cases. In these cases, the amount of cfDNA in the fetus is not enough to read the NIPT result, called "unreportable" or "inconclusive", at which point the pregnant woman is usually advised to have her blood drawn and retest. This causes inconvenience or even anxiety for pregnant women, and increases the cost of sequencing and labor for laboratories.
Low FF was investigated to find out the factors that can influence with the desire to minimize this situation to improve the value of the test. The factors associated with low fetal fractions are known: gestational age, maternal obesity, and technical problems.
II. SOME FACTORS THAT CAN CAUSE NIPT RESULTS
1. Gestational age
From the 5th week of gestation, fetal cfDNA was detectable in the mother's blood, but the amount was too low to warrant NIPT testing. From 10 to 21 weeks of gestation, FF fluctuated around 10-15%, in which fetal cfDNA increased by 0.1% per week (p<0.0001). After 21 weeks of gestation, fetal cfDNA increased by 1% per week (p<0.0001). Therefore, the recommended gestational week to perform NIPT is from the 10th week, accordingly, blood samples taken at an earlier gestational week may cause the FF to be insufficient to ensure the accuracy of the test.
Results from recent studies have shown a correlation between fetal cfDNA levels and gestational age, with FF being proportional to gestational age. In the 2020 study by Danielius et al., the team performed a retrospective study of nearly 1,000 NIPT samples, the results showed that fetal cfDNA levels gradually increased over the weeks of gestation, from 10 to 21 weeks of gestation. , FF fluctuates in the range of 10-15% (Figure 2).
Therefore, the NIPT test should only be performed between 10 and 20 weeks of pregnancy, when the cfDNA concentration is sufficient to ensure the most accurate test results as well as full consultation from the doctor.
2. Maternal obesity
Among the factors that can influence FF, a high maternal body mass index (BMI) is a well-recognized factor. Studies indicate an inverse relationship between BMI and FF to explain inconclusive NIPT results. The reason is explained that in pregnant women with obesity, total cfDNA levels are increased due to increased adipose tissue necrosis and increased apoptosis of vascular stromal cells. Although fetal cfDNA was unaffected, maternal total cfDNA was increased, resulting in a lower overall FF.
The study of Danielius et al in 2020 evaluated the correlation between maternal weight and FF (r = −0.330, p < 0.001). The results showed that the group of pregnant women weighing ≥95 kg had the lowest FF (5.6%), much lower than the group of pregnant women weighing 45-54 kg (FF was 11.1%). (Figure 2).

In addition, some factors such as maternal cancer or autoimmune disease have also been reported to affect FF, since the use of drugs to treat the disease increases the number of total DNA fragments. maternal circulation, resulting in lower FF.
3. Technical problems
Technical issues such as cfDNA extraction efficiency, library generation efficiency and genetic sequencing technology platform are factors that contribute to the success of the assay. Through the DNA extraction process, if the technique is not guaranteed, it can reduce the concentration of cfDNA in the plasma, thereby affecting the quality of gene library creation and the number of copies of sequenced DNA.
Up to now, major research centers around the world are always trying to find and come up with new technological solutions to overcome the risks that cause NIPT results to go unreported, such as improving the extraction efficiency of cfDNA. , optimize the FF to the minimum that can perform the test or set up a dedicated bioinformatics software to analyze the results.
As a pioneer in genetic analysis in Vietnam, GENTIS uses next-generation gene sequencing technology on the NextSeq 550 sequencer system of Illumina, USA. Illumina is known as a leading technology company in the world in gene sequencing, with outstanding quality voted by the worldwide community of scientists as a technology company used in scientific research, develops tests in the field of molecular biology the most.
 When comparing Illumina's technology platform with some other technology platforms, Illumina shows outstanding advantages in terms of cfDNA detection at low concentrations of 1.4 - 2.7% and test failure rate. (0.1%) lowest among the platforms being used today. In addition, the GENTIS R&D team has successfully developed a proprietary bioinformatics software that optimizes the readability of the results, thereby improving the accuracy of the NIPT test.
When comparing Illumina's technology platform with some other technology platforms, Illumina shows outstanding advantages in terms of cfDNA detection at low concentrations of 1.4 - 2.7% and test failure rate. (0.1%) lowest among the platforms being used today. In addition, the GENTIS R&D team has successfully developed a proprietary bioinformatics software that optimizes the readability of the results, thereby improving the accuracy of the NIPT test.
The NIPT test is gaining popularity today because of its high accuracy and helps to reduce the risks posed by invasive methods. However, NIPT is still a screening test, so it is essential to understand and fully understand the nature of the test, as well as the possible causes that lead to NIPT cases not determining the results.
Through the article, GENTIS hopes to have provided more useful information on factors affecting low FF, the main cause of underreported results, thereby helping clinicians make informed decisions. receive the most appropriate advice and guidance.
References:
[1] Hu P, Liang D, Chen Y, Lin Y, Qiao F, Li H, et al. An enrichment method to increase cell-free fetal DNA fraction and significantly reduce false negatives and test failures for non-invasive prenatal screening: a feasibility study. J Transl Med 2019;17:124. 29
[2] Van Opstal D, Srebniak MI, de Vries F, Govaerts LCP, Joosten M, et al. False negative NIPT results: risk figures for chromosomes 13, 18 and 21 based on chorionic villi results in 5967 cases and literature review. PLoS One 2016;11: e0146794.
[3] Scheffer PG, Wirjosoekarto SAM, Becking EC, et al. Association between low fetal fraction in cell-free DNA testing and adverse pregnancy outcome: A systematic review. Prenat Diagn. 2021;41(10):1287-1295. doi:10.1002/pd.6028
[4] Serapinas D, Boreikaitė E, Bartkevičiūtė A, Norvilaitė K, Narbekovas A, Bartkevičienė D. The Level of Free Fetal DNA as Precise Noninvasive Marker for Chromosomal Aneuploidies: First Results from BALTIC Region. Medicina (Kaunas). 2020;56(11):579. Published 2020 Oct 30. doi:10.3390/medicina56110579
[5] White K, Wang Y, Kunz LH, Schmid M. Factors associated with obtaining results on repeat cell-free DNA testing in samples redrawn due to insufficient fetal fraction [published online ahead of print, 2019 Mar 27]. J Matern Fetal Neonatal Med. 2019;1-6. doi:10.1080/14767058.2019.1594190
[6] Du Y, Chen A, Yang R, et al. A proof-of-concept study on the effects of low total cfDNA content and solutions to increase the NIPT trisomy 21 detection rate. J Clin Lab Anal. 2020;34(2):e23035. doi:10.1002/jcla.23035











