-
- 1800 2010
- EN

News
Array
(
[0] => stdClass Object
(
[id] => 1411
[id_crawler] =>
[category_product] => NULL
[thumbnail] => huyen.8.2021/600x400-web-eurofins-gentis-x-xanh-sm.jpg
[album] =>
[url_video] =>
[is_status] => 1
[is_featured] => 0
[is_form] => 0
[displayed_time] => 2026-02-03
[program] => 0
[number] => 1
[viewed] => 0
[type] =>
[type_career] =>
[level] =>
[address] =>
[address_career] =>
[expiration_time] => 0000-00-00
[created_time] => 2026-02-09 13:18:40
[updated_time] => 2026-02-27 13:50:39
[files] =>
[salary] =>
[time] =>
[created_by] => 1
[is_table_content] => 0
[language_code] => en
[slug] => Travel-smarter-save-more-on-medical-visits-with-Eurofins-Gentis-and-Xanh-SM
[title] => Travel smarter, save more on medical visits with Eurofins Gentis and Xanh SM
[description] => To bring greater convenience and cost savings to customers traveling for medical check-ups and testing, Eurofins Gentis has partnered with the Xanh SM mobility app to launch a special promotion for customers commuting to Eurofins Gentis and its nationwide network of medical partners.
[content] => 
Exclusive offers for Eurofins Gentis customers
Customers using the Xanh SM app to travel to Eurofins Gentis and partner locations will receive the following promo codes:
- 02 codes with 15% discount for Xanh SM Bike and Xanh SM Bike Plus services
- 02 codes with 12% discount for Xanh SM Car, Xanh SM Car Premium, and Xanh SM Limo services
- New Xanh SM users receive an additional 20% discount code
In addition, customers who undergo the NIPT prenatal screening test at Eurofins Gentis will receive 01 extra 20% discount code, applicable for nationwide travel with a maximum discount of VND 50,000. The promo code will be included in the leaflet attached to the hard-copy test result.
How to receive and use the promo codes
Getting your discount is simple:
- Open the Xanh SM app.
- Enter your pick-up location and destination as Eurofins Gentis or one of its partner facilities.
- The promo codes will automatically appear in the “Promotions” section.
- Select the applicable code and proceed with your ride booking.
17 participating locations under the Eurofins Gentis program
- Andrology and fertility hospital of Hanoi
- The Vietnam-Belgium Andrology & Infertility Specialist hospital
- Phenikaa University hospital
- Hong Ngoc general hospital
- Hong Ngoc - Phuc Truong Minh general hospital
- Bao Son general hospital
- Hanoi general hospital
- Phuong Dong general hospital
- An Viet general hospital
- Dong Do hospital
- Vietnam’s premier medical
- Hanh Phuc international hospital
- Saigon Reproductive Medicine and Andrology hospital
- Pham Nhat Infertility and IVF Clinic
- City international hospital
- Xuyen A general hospital
Supporting your healthcare journey
Through the collaboration between Eurofins Gentis and Xanh SM, customers can feel confident not only in the quality of medical services but also in receiving optimal transportation support—making medical visits and testing more convenient, cost-effective, and stress-free.
Book your Xanh SM ride today and experience smart mobility when visiting Eurofins Gentis and its trusted medical partners.
[content_more] => [meta_title] => Travel smarter, save more on medical visits with Eurofins Gentis and Xanh SM [meta_description] => To bring greater convenience and cost savings to customers traveling for medical check-ups and testing, Eurofins Gentis has partnered with the Xanh SM mobility app to launch a special promotion for customers commuting to Eurofins Gentis and its nationwide [meta_keyword] => GENTIS,XanhSM [thumbnail_alt] => [post_id] => 1411 [category_id] => 4 ) [1] => stdClass Object ( [id] => 1410 [id_crawler] => [category_product] => NULL [thumbnail] => banner/600x400-web-banner-thang-genneva-2.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 0 [displayed_time] => 2026-02-02 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-02-09 10:33:53 [updated_time] => 2026-02-27 13:50:48 [files] => [salary] => [time] => [created_by] => 1 [is_table_content] => 0 [language_code] => en [slug] => Prosperous-Tet-auspicious-deals-with-GenEva-Enjoy-up-to-VND-3,000,000-in-benefits-for-expecting-mothers [title] => Prosperous Tet – auspicious deals with GenEva: Enjoy up to VND 3,000,000 in benefits for expecting mothers [description] => On the occasion of welcoming the Lunar New Year 2026, Eurofins Gentis would like to extend our warmest wishes of health, peace, and a fulfilling pregnancy to all expecting mothers and their families. With the desire to accompany mothers on a healthy pregnancy journey, GenEva is launching a special early-year promotion with total benefits of up to VND 3,000,000 for customers who register for the non-invasive prenatal screening (NIPT) test. [content] =>
GenEva new year promotion details
During the promotional period, mothers who register for the GenEva NIPT test will enjoy attractive incentives—helping reduce costs while ensuring international-standard testing quality from Eurofins Gentis.
Receive up to VND 3,000,000 in cash incentives when booking:
- 9 Autosomal Recessive Single-Gene Disease Screening Package
- 18 Autosomal Recessive Single-Gene Disease Screening Package
(Incentives are applied correspondingly to each testing package.)
⏰ Promotion period: From January 26, 2026, until further notice.
Early registration is encouraged as promotional slots are limited.
This program applies to customers who book within the promotional period, subject to availability.
The offer aims to support expectant mothers in accessing advanced prenatal screening services early—empowering them to proactively monitor and manage pregnancy health from the very first stages.
Why choose GenEva NIPT?
GenEva non-invasive prenatal testing (NIPT) analyzes cell-free fetal DNA present in the mother’s blood to assess the risk of common chromosomal abnormalities.
- Safe for both mother and baby
- Non-invasive procedure
- High accuracy
GenEva is implemented at Eurofins Gentis with a rigorous testing process, an experienced team of experts, and internationally accredited laboratory systems.
Eligible participants
The GenEva New Year 2026 promotion applies to pregnant women who wish to undergo NIPT prenatal screening and register for the service within the promotional period in accordance with Eurofins Gentis’ terms and conditions.
How to register and receive the promotion
For detailed consultation and to book your GenEva NIPT test, please contact our hotline at 0988 00 2010. The Eurofins Gentis expert team is ready to assist mothers in selecting the most suitable testing package and guide them through a quick and convenient process.
[content_more] => [meta_title] => Prosperous Tet – auspicious deals with GenEva: Enjoy up to VND 3,000,000 in benefits for expecting m [meta_description] => On the occasion of welcoming the Lunar New Year 2026, Eurofins Gentis would like to extend our warmest wishes of health, peace, and a fulfilling pregnancy to all expecting mothers and their families. With the desire to accompany mothers on a healthy pregn [meta_keyword] => GENTIS,NIPT [thumbnail_alt] => [post_id] => 1410 [category_id] => 4 ) [2] => stdClass Object ( [id] => 1409 [id_crawler] => [category_product] => NULL [thumbnail] => banner/600x400-web--banner-thang-2.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 1 [displayed_time] => 2026-02-02 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-02-09 10:26:42 [updated_time] => 2026-02-27 13:50:56 [files] => [salary] => [time] => [created_by] => 1 [is_table_content] => 1 [language_code] => en [slug] => New-Year-2026-promotion-20%-off-all-DNA-testing-services-at-Eurofins-Gentis [title] => New Year 2026 promotion – 20% off all DNA testing services at Eurofins Gentis [description] => At the beginning of 2026, Eurofins Gentis is launching a special promotion offering 20% off all DNA testing services, providing customers with a cost-saving opportunity to access internationally standardized, accurate, and confidential DNA testing services. [content] =>On the occasion of the New Year, Eurofins Gentis sincerely wishes our valued customers good health, prosperity, and happiness. We deeply appreciate your continued trust and companionship over the years. As we enter 2026, Eurofins Gentis remains committed to enhancing service quality and delivering accurate, scientific, internationally accredited DNA testing solutions that help protect the health and family values of Vietnamese families.
As a token of appreciation, Eurofins Gentis is pleased to offer 20% off all DNA testing services.
The promotion is valid from January 26, 2026, until further notice, with priority given to early registrations. To receive the offer, customers may contact the hotline 0988.00.2010 for consultation and appointment scheduling.

List of discounted DNA testing services
Eurofins Gentis provides a wide range of DNA testing services to meet different customer needs:
Voluntary DNA testing
- Paternity and maternity testing
- Sibling testing (half-siblings, full siblings)
- Paternal lineage testing (grandfather–grandchild, uncle–nephew/niece, etc.)
- Maternal lineage testing
Legal DNA testing
- Birth certificate support
- Determination of child support obligations after divorce
- Relationship confirmation for inheritance and other legal procedures
- Identification of biological relationships to reconnect with family members
Non-Invasive prenatal DNA testing (NIPT)
This service is also eligible for the 20% discount, with fast turnaround time—typically within 7–10 days.
Sample collection options
Customers may choose one of the following sample submission methods:
- Schedule home sample collection via hotline (available from Da Nang northward).
- Self-collect samples and send them to Gentis Testing Center at:
3rd Floor, V+ Commercial Center, 505 Minh Khai, Vinh Tuy, Hanoi. - Visit the Gentis Testing Center directly for staff-assisted sample collection.
Accepted sample types include blood, buccal swabs (oral mucosa), hair with roots, fingernails/toenails, umbilical cord samples, and special samples such as teeth, cigarette butts, toothbrushes, chewing gum, clothing, and more.
Important notes when registering
The promotion applies to customers who register online and is available for a limited time. To enjoy the 20% discount on all DNA testing services, customers are encouraged to contact Eurofins Gentis early for detailed consultation and step-by-step guidance on the testing process.
Start the New Year with confidence and peace of mind—let Eurofins Gentis accompany you in protecting your family’s truth and future.
[content_more] => [meta_title] => New Year 2026 promotion – 20% off all DNA testing services at Eurofins Gentis [meta_description] => At the beginning of 2026, Eurofins Gentis is launching a special promotion offering 20% off all DNA testing services, providing customers with a cost-saving opportunity to access internationally standardized, accurate, and confidential DNA testing service [meta_keyword] => ADN [thumbnail_alt] => [post_id] => 1409 [category_id] => 4 ) [3] => stdClass Object ( [id] => 1408 [id_crawler] => [category_product] => NULL [thumbnail] => c297db27af2f2171783e.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 0 [displayed_time] => 2026-02-06 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-02-06 09:54:55 [updated_time] => 2026-02-27 13:51:10 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => Summary-of-5-hereditary-cancer-testing-packages-at-Eurofins-GENTIS [title] => Summary of 5 hereditary cancer testing packages at Eurofins GENTIS [description] => Cancer is one of the leading causes of death worldwide. Numerous studies indicate that 5–10% of cancer cases are associated with hereditary factors, resulting from gene mutations that can be passed down from one generation to the next. Hereditary cancer testing helps identify risks early, enabling the development of appropriate monitoring, prevention, and medical intervention strategies. [content] =>
Understanding the growing demand for genetic screening in the community, Eurofins GENTIS has developed five hereditary cancer testing packages ranging from basic to advanced levels, tailored to different individuals, age groups, and family histories.
Why consider hereditary cancer testing?
Unlike conventional tests that detect cancer after it develops, hereditary cancer testing focuses on genetic analysis to determine cancer risk before clinical symptoms appear.
Key benefits include:
- Early detection of potential hereditary cancer risks
- Proactive development of personalized screening plans
- Support for physicians in prevention and treatment counseling
- Provision of valuable genetic information for family members
- Reduced psychological burden through better understanding of personal health risks
With internationally standardized laboratories and an experienced team of genetic experts, Eurofins GENTIS delivers accurate, reliable testing solutions tailored to the Vietnamese population.

Summary of 5 hereditary cancer testing packages at Eurofins GENTIS
LADY - CARE: Hereditary cancer testing package for women
LADY - CARE is specifically designed for women, focusing on cancers with high incidence rates and strong hereditary associations.
- Analysis of 10 genes
- Screening for hereditary breast cancer, ovarian cancer, and colorectal cancer
Suitable for:
- Women with a family history of breast, ovarian, or colorectal cancer
- Women who wish to proactively assess hereditary cancer risk
- Women of reproductive or middle age
LADY - CARE helps women better understand their genetic risks and develop appropriate medical monitoring and intervention plans to enhance quality of life.
MEN - CARE: Hereditary cancer screening solution for Men
Men also face various cancers influenced by hereditary factors, particularly prostate and gastrointestinal cancers.
- Analysis of 10 genes
- Screening for hereditary prostate cancer, colorectal cancer, and gastric cancer
Suitable for:
- Men with a family history of related cancers
- Middle-aged men or those with high-risk lifestyles or environmental exposures
- Individuals seeking proactive genetic health screening
MEN - CARE is an important step in early cancer risk detection, enabling long-term prevention and medical follow-up.
PRE - CARE: Expanded screening for 15 hereditary cancers
PRE - CARE is an extended hereditary cancer testing package for individuals seeking more comprehensive risk assessment.
- Analysis of mutations in 17 genes
- Screening for 15 hereditary cancers, including: lung, stomach, breast, ovarian, colorectal, pancreatic, kidney, prostate, thyroid, skin, endometrial cancers, paraganglioma, retinoblastoma, pheochromocytoma, and multiple endocrine neoplasia
Suitable for:
- Individuals with family histories of multiple cancer types
- Those seeking expanded hereditary cancer screening
- Individuals planning marriage or childbirth
PRO - CARE: Advanced hereditary cancer testing package
PRO - CARE provides a comprehensive overview of hereditary cancer risks.
- Analysis of mutations in 133 genes
- Screening for 30 hereditary cancers, including: lung, stomach, breast, ovarian, colorectal, pancreatic, kidney, prostate, thyroid, skin, endometrial, liver, bladder, head and neck, esophageal, biliary tract, small intestine, brain, bone, parathyroid cancers; acute leukemia (blood cancer); rhabdomyosarcoma; pituitary adenoma; neuroblastoma; neurofibroma; and multiple myeloma
Suitable for:
- Individuals at high risk of cancer
- Families with multiple affected members
- Those requiring comprehensive genetic evaluation for long-term monitoring
DIAMOND - CARE: The most comprehensive genetic testing package
DIAMOND - CARE is the most advanced and comprehensive package at Eurofins GENTIS, combining hereditary cancer screening with inherited chronic disease testing.
- Total analysis of 177 genes associated with 69 hereditary chronic diseases and cancers, including:
- 10 genes – 9 common autosomal recessive disorders:
Alpha Thalassemia, Beta Thalassemia, G6PD deficiency, Phenylketonuria (PKU), Galactose metabolism disorder, Citrin deficiency–related cholestasis, 5-Alpha Reductase deficiency–related male sex development disorder, Pompe disease, Wilson disease - 133 genes – Screening for 30 hereditary cancers (entire PRO - CARE panel)
- 34 genes – 30 hereditary syndromes and chronic conditions:
20 cardiovascular diseases, 5 connective tissue disorders, and 5 endocrine–metabolic disorders
DIAMOND - CARE is especially suitable for individuals seeking a comprehensive evaluation of hereditary health risks for themselves and their families.
With advanced gene sequencing technology, strict quality control processes, and a highly experienced team of experts, Eurofins GENTIS is committed to delivering accurate, reliable, and clinically valuable results.
Hereditary cancer testing is not just a test — it is a proactive step toward early prevention, appropriate monitoring, and personalized healthcare.
Contact Eurofins GENTIS via hotline 1800 2010 for detailed consultation and to choose the most suitable testing package for you and your family.
[content_more] => [meta_title] => Summary of 5 hereditary cancer testing packages at Eurofins GENTIS [meta_description] => Cancer is one of the leading causes of death worldwide. Numerous studies indicate that 5–10% of cancer cases are associated with hereditary factors, resulting from gene mutations that can be passed down from one generation to the next. Hereditary cancer t [meta_keyword] => GENTIS,genetic disorder [thumbnail_alt] => [post_id] => 1408 [category_id] => 4 ) [4] => stdClass Object ( [id] => 1406 [id_crawler] => [category_product] => NULL [thumbnail] => img_4684.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 0 [displayed_time] => 2026-02-06 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-02-06 08:41:18 [updated_time] => 2026-02-07 09:25:41 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => Eurofins-GENTIS's-year-end-party-2025-United-as-one-steadfast-forward [title] => Eurofins GENTIS's year end party 2025 - United as one, steadfast forward [description] => Eurofins GENTIS solemnly hosted the Year End Party 2025 with the theme “United as One, Steadfast Forward.” The event was not only an occasion to review and reflect on a year of development, but also a meaningful space for connection, where the entire Eurofins GENTIS family gathered, shared, and spread the spirit of unity and determination toward the future. [content] =>
Year End Party 2025 took place in an atmosphere that was both formal and warm, vibrant yet heartfelt, leaving many emotions and lasting impressions on every employee, partner, and expert in attendance.

Looking back at 2025 – A year of effort and achievement
The year 2025 marked a period of strong growth for Eurofins GENTIS with many important milestones. The achievements were not only reflected in growth figures, but also in improvements in service quality, brand reputation, and the professional working spirit of the entire team.
The Year End Party was an opportunity for the Eurofins GENTIS collective to look back on that journey — a journey built on perseverance, responsibility, and unity from every individual. Each member, in different roles, contributed to a proud overall picture that reflects the company’s sustainable development.

Eurofins GENTIS highly appreciates and values the presence of our esteemed partners and experts at the Year End Party 2025. Their attendance was not only an honor, but also a testament to the strong, trustworthy, and long-term partnerships between Eurofins GENTIS and its collaborators.
The trust and support from our partners and experts have played a vital role in the successes Eurofins GENTIS achieved throughout 2025. The party was an occasion for all sides to reflect on the journey so far, while opening new expectations for deeper and more effective cooperation in the future.

One of the most memorable and emotional moments of the program was the group photo of Eurofins GENTIS employees together with partners and experts. In a space filled with joy and pride, every smile and every glance reflected unity, connection, and confidence in the road ahead.
The photo not only captured a moment, but also became a symbol of the message “United as One, Steadfast Forward” — when everyone shares the same goals, looks toward the future, and is ready to work together to build a stronger Eurofins GENTIS.

At the Year End Party 2025, Mr. Do Manh Ha – General Director of Eurofins GENTIS — delivered an important speech sharing the company’s outstanding achievements in 2025. These results clearly demonstrate the right strategies, the determination of the leadership team, and the tireless efforts of all employees.
In addition to reflecting on the past year, the General Director also affirmed the vision, goals, and development directions for 2026, with expectations to further improve service quality, expand cooperation, and strengthen Eurofins GENTIS’s position in its field of expertise. His sharing was not only strategic in orientation but also highly inspiring, motivating the entire team to enter the new year with greater confidence and determination.

A meaningful and significant part of the Year End Party 2025 was the ceremony honoring outstanding individuals and teams. These were the faces that made remarkable contributions, demonstrating responsibility, creativity, and continuous effort throughout the year.






Each name called was a story of dedication, a testament to wholehearted commitment and the aspiration to grow. The enthusiastic applause was not only well-deserved recognition but also a great source of encouragement, spreading positivity and promoting a culture of recognition and appreciation at Eurofins GENTIS.
After the formal moments, the program moved into the year-end banquet in a lively, cozy, and joyful atmosphere. New Year wishes and shared stories helped erase distances and brought members closer together.
Additionally, awards for outstanding activities and contributions were not only a form of recognition but also motivation for all employees to continue being creative, striving, and accompanying Eurofins GENTIS on its development journey ahead.



.jpg)


An indispensable part of the Year End Party 2025 was the exciting Lucky Draw. The suspenseful waiting moments and the bursts of cheers when attractive prizes found their winners made the atmosphere even more energetic.
More than just prizes, the Lucky Draw was an occasion for everyone to share joy, create memorable moments, and strengthen the bonds among members of the Eurofins GENTIS family.
Closing a memorable year, ready for a new journey
Year End Party 2025 – “United as One, Steadfast Forward” concluded in a joyful, warm, and emotional atmosphere. The year 2025 left many proud marks, serving as a strong foundation for Eurofins GENTIS to enter 2026 with greater confidence, ambition, and determination.

With a spirit of unity, solidarity, and continuous effort from the entire team, Eurofins GENTIS will continue moving forward together, reaching new heights and building a more sustainable and brilliant future in the years to come.
[content_more] => [meta_title] => Eurofins GENTIS's year end party 2025 - United as one, steadfast forward [meta_description] => Eurofins GENTIS solemnly hosted the Year End Party 2025 with the theme “United as One, Steadfast Forward.” The event was not only an occasion to review and reflect on a year of development, but also a meaningful space for connection, where the entire Euro [meta_keyword] => GENTIS,YEAR END PARTY [thumbnail_alt] => [post_id] => 1406 [category_id] => 4 ) [5] => stdClass Object ( [id] => 1405 [id_crawler] => [category_product] => NULL [thumbnail] => z7475342255361_6d2a37fa1d1528705dc55057db6a4222.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 1 [displayed_time] => 0000-00-00 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-01-29 13:20:52 [updated_time] => 2026-01-30 13:30:18 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => Eurofins-GENTIS-and-Phenikaa-hospital-strengthen-professional-exchange-on-preimplantation-genetic-testing [title] => Eurofins GENTIS and Phenikaa hospital strengthen professional exchange on preimplantation genetic testing [description] => In recent years, alongside in vitro fertilization (IVF), preimplantation genetic testing (PGT) solutions have increasingly been recognized as a key factor in improving success rates, minimizing risks, and working toward the goal of delivering healthy babies. Scientifically guided embryo selection plays a crucial role in helping clinicians and patients make safer and more effective decisions in each treatment cycle. [content] =>
Responding to the growing demand for the application of PGT solutions in assisted reproduction, Eurofins GENTIS recently collaborated with Phenikaa Hospital to organize a professional working session. The meeting focused on exchanging insights and updating advanced genetic testing solutions currently applied in reproductive medicine, including specialized technologies and services developed and implemented by Eurofins GENTIS.
Representing Eurofins GENTIS, Dr. Nguyen Quang Vinh (Laboratory Director) directly presented and reported on the professional content at the session. On the Phenikaa Hospital side, the meeting was attended by a team of physicians, specialists, and technicians in assisted reproduction, who engaged in in-depth discussions on the practical application of genetic testing in IVF treatment.

At the meeting, Dr. Vinh provided an overview of the PGT testing system currently implemented at Eurofins GENTIS, with a focus on PGT-A/SR—preimplantation genetic testing for aneuploidies and structural rearrangements. PGT-A/SR enables comprehensive analysis of all 24 chromosomes, detecting numerical and structural abnormalities, including aneuploidy, deletions, duplications, and mosaicism. The application of PGT-A/SR helps IVF clinicians select embryos with higher implantation potential, reduce the risk of early miscarriage, and increase embryo transfer success rates, particularly in advanced-age patients, those with repeated IVF failures, or a history of pregnancy loss. Data shared by Eurofins GENTIS showed that embryos with chromosomal abnormalities account for nearly half of all analyzed samples. This highlights the importance of preimplantation genetic testing, as many embryos with good morphology may still harbor underlying genetic abnormalities that can directly affect pregnancy outcomes if not screened early.
In addition to PGT-A/SR, PGT-M was another topic of particular interest to Phenikaa Hospital. This solution is designed for couples who carry genes associated with inherited diseases but wish to have healthy children free from these conditions. Eurofins GENTIS has currently implemented more than 100 PGT-M protocols for a wide range of conditions such as thalassemia, spinal muscular atrophy (SMA), hemophilia, and other rare genetic disorders, using advanced technologies including next-generation sequencing (NGS), STR, SNP analysis, and karyomapping. The integration of PGT-M with IVF helps prevent genetic diseases at the embryo stage, delivering long-term benefits for the health of future generations.
Within the framework of the session, Dr. Vinh also highlighted Eurofins GENTIS’s testing capacity and internationally standardized laboratory system, featuring the Illumina MiSeq NGS platform and specialized analysis software BlueFuse. Notably, the achievement of CAP accreditation for PGT testing serves as strong evidence of quality, reliability, and standardization across the entire genetic testing workflow at GENTIS.
Beyond introducing genetic testing services and modern laboratory infrastructure, Dr. Nguyen Quang Vinh also directly addressed professional questions from Phenikaa Hospital’s experts and physicians, helping clarify practical issues related to the application of genetic testing in assisted reproduction. Physicians at Phenikaa Hospital highly appreciated the professional insights, real-world data, and testing solutions provided by Eurofins GENTIS, considering them an important foundation for improving treatment outcomes and the quality of care for IVF patients.
The professional exchange between Eurofins GENTIS and Phenikaa Hospital not only held academic value but also opened up directions for sustainable collaboration in training, clinical consultation, and the implementation of advanced genetic testing solutions. Through close cooperation, both parties expect to contribute to improving IVF treatment effectiveness, reducing the psychological and financial burden on patients, and delivering meaningful value to the community in the journey toward fulfilling the dream of having healthy children.
[content_more] => [meta_title] => Eurofins GENTIS and Phenikaa hospital strengthen professional exchange on preimplantation genetic te [meta_description] => In recent years, alongside in vitro fertilization (IVF), preimplantation genetic testing (PGT) solutions have increasingly been recognized as a key factor in improving success rates, minimizing risks, and working toward the goal of delivering healthy babi [meta_keyword] => GENTIS,Phenikaa [thumbnail_alt] => [post_id] => 1405 [category_id] => 4 ) [6] => stdClass Object ( [id] => 1404 [id_crawler] => [category_product] => NULL [thumbnail] => rabbit_media-4214.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 0 [displayed_time] => 2026-01-23 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-01-23 10:43:46 [updated_time] => 2026-01-30 13:27:57 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => Trends-and-applications-of-AI-and-omics-data-in-genetic-testing-and-reproductive-genetic-counseling [title] => Trends and applications of AI and omics data in genetic testing and reproductive genetic counseling [description] => In recent years, artificial intelligence (AI) has emerged as one of the key driving forces behind the technological revolution across many fields, especially healthcare. From medical imaging diagnostics and chronic disease management to drug research and development, AI has demonstrated its pivotal role in improving treatment effectiveness and personalizing healthcare. In the field of genetics and assisted reproduction, the integration of AI, bioinformatics, and Omics data is expected to create major breakthroughs, significantly enhancing the quality of screening, diagnosis, and genetic counseling. [content] =>AI and omics data – The foundation of precision medicine
The rapid development of next-generation sequencing (NGS), mass spectrometry technologies, along with the increasing capacity for collecting and storing large-scale clinical data, has generated an enormous volume of information in medical genetics. Omics data—including genomics, transcriptomics, proteomics (proteins and post-translational modifications), metabolomics (metabolites and hormones), together with bioinformatics and AI—provide a comprehensive reflection of biological characteristics at the molecular level. However, analyzing and effectively exploiting these datasets poses a major challenge if relying solely on traditional methods.
 Dr. Pham Dinh Minh (R&D Director, Eurofins GENTIS) presenting at the Symposium on Reproductive Genetics and PGT
Dr. Pham Dinh Minh (R&D Director, Eurofins GENTIS) presenting at the Symposium on Reproductive Genetics and PGT
With its machine learning capabilities and multidimensional data analysis, AI enables rapid and accurate processing of complex Omics datasets, thereby uncovering potential relationships among genetic factors, clinical data, and disease risks. In assisted reproduction, this opens up opportunities to improve embryo screening quality, predict implantation potential, and provide more scientific and personalized genetic risk counseling for couples.
Eurofins GENTIS pioneers the application of AI in reproductive genetics
According to Dr. Pham Dinh Minh (R&D Director of Eurofins GENTIS) at the Symposium on Reproductive Genetics and PGT, in recent years Eurofins GENTIS has focused on developing information technology platforms and AI applications integrated with Omics data to support genetic screening, counseling, and management of hereditary diseases. These solutions not only provide professional support for hospitals and partners but also strengthen the connection between Eurofins GENTIS, clinicians, healthcare facilities, and patients, contributing to improved user experience and healthcare service quality.
In the field of genetic analysis, Eurofins GENTIS has been researching and developing various bioinformatics tools to support the application of AI in studying the molecular mechanisms of fertilization, embryo development, and factors affecting infertility and subfertility. These studies aim to improve the success rates of in vitro fertilization (IVF) and to personalize treatment for each patient in the future.
PGT-AI – A new approach to embryo selection
One of the greatest challenges in IVF today is how to increase pregnancy rates and the likelihood of delivering healthy babies after embryo transfer. According to Dr. Pham Dinh Minh, the embryo is the decisive factor determining the success of an IVF cycle. Selecting the best embryo not only affects implantation rates but also has long-term implications for the child’s future health.
In this context, the application of artificial intelligence in preimplantation genetic testing (PGT-AI) is considered a highly promising trend. PGT-AI goes beyond the assessment of chromosomal abnormalities in embryos and aims to select the “best embryo among good embryos” from a genetic perspective.
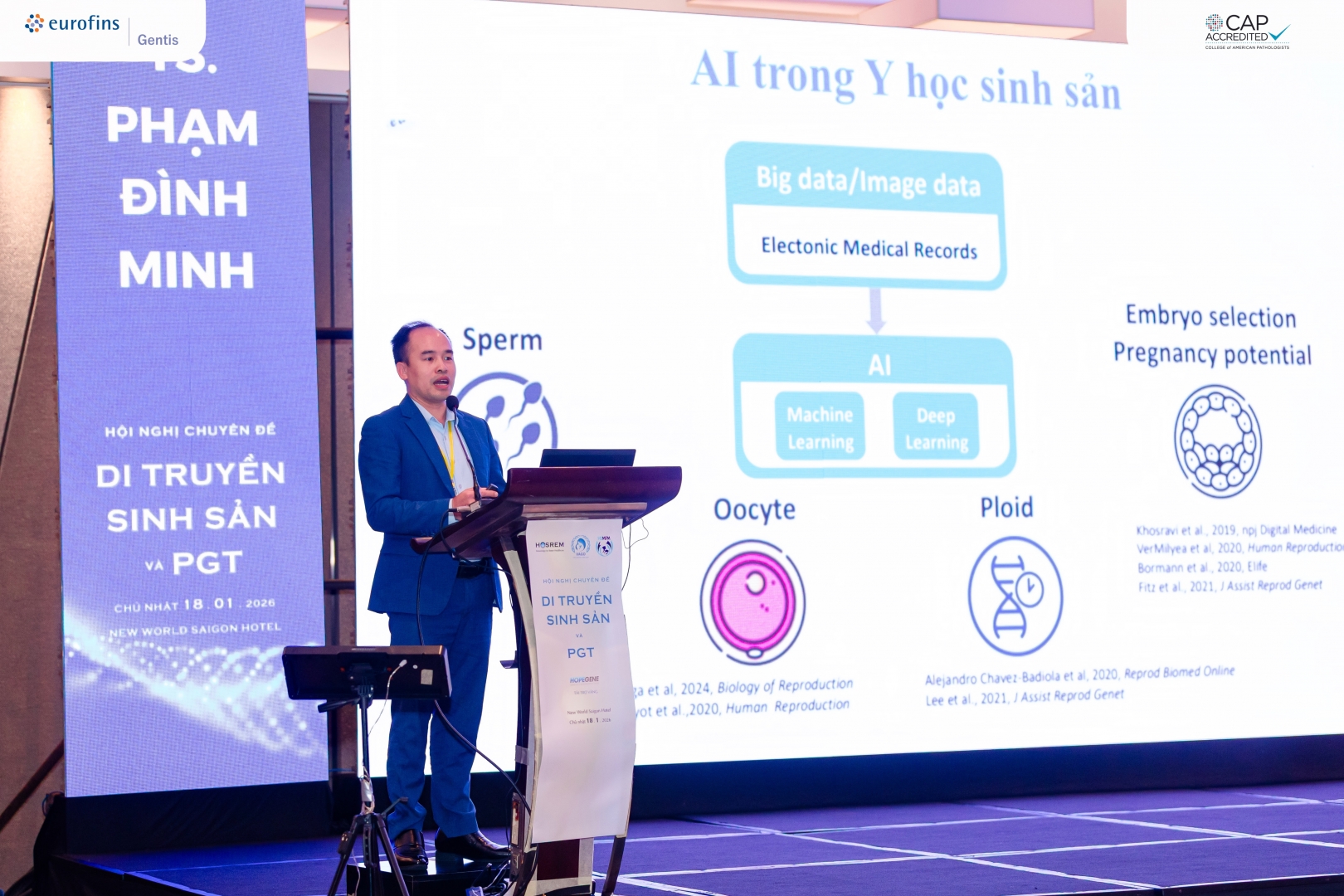
Eurofins GENTIS Is researching the application of AI to predict embryo transfer success
Currently, Eurofins GENTIS is conducting pilot studies on applying AI to predict the success potential of embryo transfer. By analyzing the genetic data of embryos in combination with maternal clinical factors, AI can help identify relationships between embryos and their compatibility with the uterine environment. This enables clinicians to make optimal embryo selection decisions, thereby improving implantation rates and overall IVF success.
By integrating maternal clinical data and expanding the analysis of embryonic genetic data within PGT-AI, the research team at Eurofins GENTIS is developing screening and predictive models to identify healthy embryos with high implantation potential. This is regarded as an important step toward personalized counseling and assisted reproductive treatment.
Applications of AI in NIPT and newborn screening
In addition to IVF and PGT-AI, AI is also being strongly applied by Eurofins GENTIS in the fields of prenatal and newborn screening. In non-invasive prenatal testing (NIPT), Eurofins GENTIS collaborates with the University of Engineering and Technology – Vietnam National University, Hanoi, to develop bioinformatics algorithms that expand the detection spectrum of genetic variants. As a result, the sensitivity, accuracy, and specificity of NIPT have been significantly improved, contributing to higher-quality fetal anomaly screening.
 Eurofins GENTIS Is researching and applying AI and Information technology in newborn screening
Eurofins GENTIS Is researching and applying AI and Information technology in newborn screening
For newborn screening, Eurofins GENTIS is developing AI-integrated software solutions to enhance the detection of complex metabolic disorders based on mass spectrometry data. From these datasets, algorithms are built to support rapid and accurate early prediction and diagnosis of genetic diseases related to metabolic disorders in newborns.
Notably, in the process of reporting results, Eurofins GENTIS has integrated information technology and AI to interpret testing data, helping clinicians and families more easily access disease-related information, understand risks, and determine appropriate treatment directions for infants.
The application of AI and Omics data in genetic testing and counseling is ushering in a new era for assisted reproduction and precision medicine. With ongoing research and real-world applications, Eurofins GENTIS demonstrates its pioneering role in bringing advanced technologies into reproductive healthcare services in Vietnam.
In the future, as datasets become more comprehensive and AI algorithms more refined, AI-based genetic solutions are expected to support clinicians in making more accurate decisions, optimizing treatment outcomes, creating opportunities for parenthood for many infertile couples, and contributing to sustainable improvements in population health.
[content_more] => [meta_title] => Trends and applications of AI and omics data in genetic testing and reproductive genetic counseling [meta_description] => In recent years, artificial intelligence (AI) has emerged as one of the key driving forces behind the technological revolution across many fields, especially healthcare. From medical imaging diagnostics and chronic disease management to drug research and [meta_keyword] => GENTIS [thumbnail_alt] => [post_id] => 1404 [category_id] => 4 ) [7] => stdClass Object ( [id] => 1403 [id_crawler] => [category_product] => NULL [thumbnail] => f28d3c7874e6fbb8a2f7.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 0 [displayed_time] => 2026-01-13 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2026-01-13 16:14:28 [updated_time] => 2026-01-19 08:30:31 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => Warning-of-fraudulent-impersonation-of-Eurofins-GENTIS-requesting-customers-to-transfer-money-for-early-delivery-of-test-results [title] => Warning of fraudulent impersonation of Eurofins GENTIS requesting customers to transfer money for early delivery of test results [description] => Dear Valued customers and partners, [content] =>Recently, Eurofins GENTIS has received reports from several customers about unknown individuals calling by phone, impersonating company staff, and requesting payment of shipping fees for the delivery of test results (for NIPT tests and newborn screening tests), claiming that the results would be sent directly to the customers’ homes.

In response to this situation, Eurofins GENTIS would like to clearly affirm that the Company does not make phone calls requesting customers to transfer money via telephone or to personal accounts to pay any fees related to the delivery of test results. All service fees (if any) are publicly and transparently communicated at the time of registration and are implemented strictly in accordance with Eurofins GENTIS procedures. At the same time, we do not sell, provide, disclose, or allow the leakage of customers’ personal information in any form.
Currently, Eurofins GENTIS uses only one official customer service hotline: 1800 2010 to contact customers and notify them of test results. Any calls from other phone numbers requesting money transfers or personal information are not from Eurofins GENTIS.
In the context of rapid digital technology development, personal information may be collected from various sources and exploited for fraudulent activities, causing inconvenience and even serious impacts on customers’ rights and property. Therefore, Eurofins GENTIS kindly asks our partners and customers to remain vigilant, not to provide personal information, OTP codes, or bank account details, and not to transfer money without proper verification of the information.
Upon receiving any suspicious calls or unusual information related to test results, partners and customers are kindly requested to contact Eurofins GENTIS directly via the hotline 1800 2010 for timely verification and support.
Eurofins GENTIS sincerely thanks our valued partners and customers for their trust and companionship over the years, and kindly asks everyone to share this information with relatives and the community to help prevent unfortunate risks that may occur.
For further information, please contact:
EUROFINS GENTIS HANOI:
3rd Floor, V+ Shopping Center, 505 Minh Khai, Vinh Tuy Ward, Hanoi
EUROFINS GENTIS HO CHI MINH CITY:
8/24 Nguyen Dinh Khoi, Tan Son Nhat Ward, Ho Chi Minh City
Hotline: 1800 2010
Email: dichvu@gentis.com.vn
Sincerely,
[content_more] => [meta_title] => Warning of fraudulent impersonation of Eurofins GENTIS requesting customers to transfer money for ea [meta_description] => Dear Valued customers and partners, [meta_keyword] => GENTIS [thumbnail_alt] => [post_id] => 1403 [category_id] => 4 ) )Travel smarter, save more on medical visits with Eurofins Gentis and Xanh SM
Prosperous Tet – auspicious deals with GenEva: Enjoy up to VND 3,000,000 in benefits for expecting mothers
New Year 2026 promotion – 20% off all DNA testing services at Eurofins Gentis
Summary of 5 hereditary cancer testing packages at Eurofins GENTIS
Eurofins GENTIS's year end party 2025 - United as one, steadfast forward
Eurofins GENTIS and Phenikaa hospital strengthen professional exchange on preimplantation genetic testing
Trends and applications of AI and omics data in genetic testing and reproductive genetic counseling
Warning of fraudulent impersonation of Eurofins GENTIS requesting customers to transfer money for early delivery of test results
Please fill in the information below to receive our supports and consultations!
Please fill in the information below to receive our supports and consultations!















