In IVF/ICSI procedures, the first and most important step is to implement a regimen of controlled ovarian stimulation (COS). Normally, in each menstrual cycle, only one follicle in the ovary develops and falls, if it meets sperm, it will fuse and form an embryo. The chance of an embryo implanting and continuing to develop is about 5 to 20%, depending on the age of the woman. The remaining follicles will degenerate, and the mature ovum will be released into the fallopian tube. Therefore, in order to increase the success rate of infertility treatment, we need to harvest more follicles. One cycle of IVF/ICSI will require 10-15 mature eggs for fertilization. [first]
So what is controlled ovarian stimulation (COS)?
Controlled Ovarian Stimulation (COS) is a method of in vitro fertilization (IVF) to increase the number of eggs produced during a reproductive cycle. This process involves the use of sex hormones to stimulate the growth of follicles in the ovaries, in order to achieve the optimal number of eggs for the purpose of implementing IVF/ICSI treatment. This procedure is closely monitored with ultrasound and hormone testing to ensure safety and effectiveness. COS is an important step in the IVF process, allowing infertile people to have a better chance of getting pregnant. Ovarian stimulation uses hormones that act on the reproductive endocrine axis to promote the development of ovarian follicles and increase the number of ovarian follicles obtained, thereby increasing the chances of fertilization and conception in a woman.
The goal of ovarian stimulation is to:
- Ensure high efficiency;
• Obtaining the optimal number of oocytes (10-15 oocytes) increases the pregnancy rate;
• Ensure safety, reduce risks and risks;
• Reduce the rate of OHSS, reduce the rate of cycle cancellation, reduce the rate of multiple pregnancies. [2]
Ovarian development:
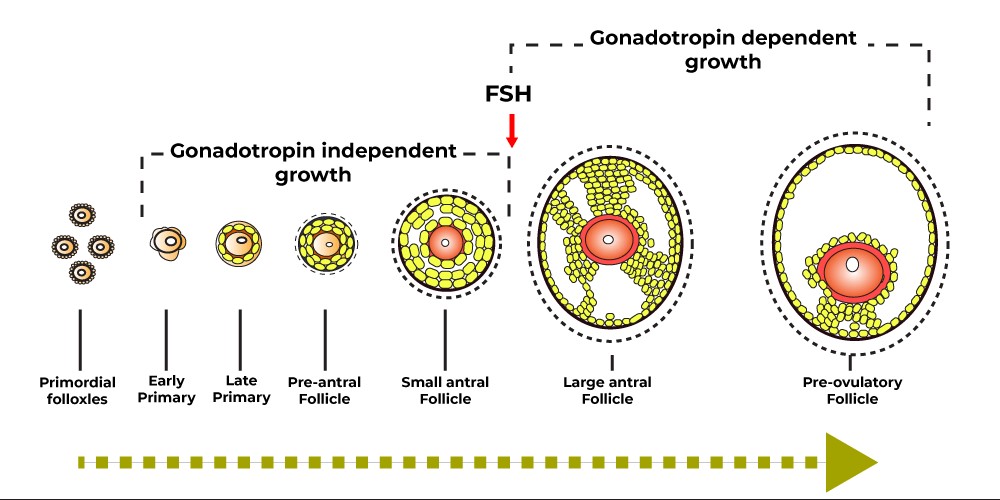
- Ovarian follicles develop in two phases: the hormone-independent phase, which lasts about 3 months before the menstrual cycle, and the FSH-dependent phase, which begins at the beginning of the menstrual cycle. [3]
- Pre-antral follicles
At the beginning of the menstrual cycle, the concentration of FSH gradually increases causing a group of follicles to develop into the primary follicular stage with a size of about 200 mm and many surrounding granulosa cells. The number of receptors on the surface of granulosa cells increases to about 1500 receptors/cell. At this point, the granulosa cells initiate the production of 17 b-estradiol by aromatization of the androgen produced by the cortical cells under the influence of LH (two-cell gonadotropic hypothesis). Estradiol and FSH promote granulosa cell proliferation and increase the number of receptors on the granulosa cell membrane. Cyst fluid begins to form and accumulates in the cyst.
- Antral follicles
The secondary cyst is about 500mm in size. On days 5-7 of the cycle, only the dominant follicles can continue to grow due to the ability to convert androgens into estrogens, while the remaining follicles will be degraded. The dominant follicle continues to grow, secreting estradiol and inhibin B, which reduces FSH secretion from the pituitary. FSH stimulates the expression of LH receptors on the surface of granulosa cells. Increasing estradiol levels stimulate the release of peak LH from the pituitary gland, which occurs when E2 levels peak at 12-14 hours. At least 33 regulators of follicular growth and regression have been identified, including ovarian peptides, of which Insulin-Like Growth factor I (IGF-I) plays an important role in promoting the process of aromatherapy, stimulates cell division of granulosa cells and increases the number of LH receptors. Inhibin inhibits the production of FSH, Activin.
- Preovulate follicle
Pre-ovulatory follicles are round and contain follicular fluid. They are attached to the follicular wall by the stalk and contain oocyte (oophoros cumulus) cells. High-frequency transducer ultrasound can show oocytes about 36 hours before ovulation. Increased estrogen levels increase LH secretion. The LH peak causes the follicle to lutealize and stimulates the granulosa cells to produce progesterone. LH stimulates the production of cytokines of which the best known is Interlekin-1 (IL-1) which has a prostaglandin and proteolytic effect that causes ovarian follicle rupture. Ovulation occurs about 34-36 hours after the peak of LH, when the follicle reaches an average size of 20 mm, the ovum in the follicle is released, moving along the fallopian tube to the uterine cavity. After ovulation, the remaining follicular cells become the corpus luteum and continue to produce estradiol, progesterone, and inhibin, which inhibit the growth of other follicles. At the end of the luteal phase, without hCG, the corpus luteum will atrophy, causing the levels of ovarian hormones to drop, causing the lining of the uterus to shed, and allowing the hormone FSH to rise gradually to begin an new ovarian cycle.
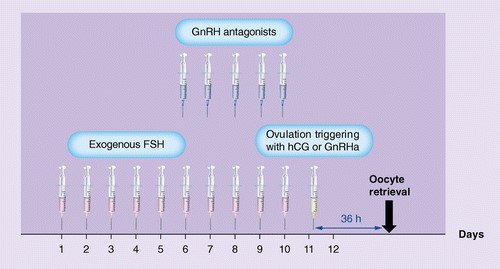
For the IVF/ICSI cycle, follicular stimulation is usually performed with recombinant FSH and a GnRH analogue to inhibit the occurrence of an early LH peak. At the end of the cycle, hCG or GnRH agonist is used to "simulate" the natural endogenous LH surge to trigger oocyte maturation and initiate ovulation. When ovarian stimulation is performed, depending on individual patient factors, results can be classified into 4 main groups [4]:
- Poor responders according to Bologna criteria: 3 oocytes obtained
- Sub-optimal responder: 4 to 9 oocytes are obtained
- Normal responder: 10 to 15 oocytes are obtained
- Hyper-responder: 15 oocytes obtained
Therefore, identifying and classifying patients is the key to choosing the correct treatment strategy.
Many factors influencing regimen choice and response to ovarian stimulation have been demonstrated, such as [5]:
- Frequency and range of GnRH . pulses
- Different isoforms of FSH and LH
-FSH receptor gene polymorphism
- Intracellular signaling
- Inter-individual differences (age, BMI, AMH, AFC and endocrine status, serum follicle-stimulating hormone-FSH basis)
In HTSS, a combination of factors can exacerbate the decrease in the concentration and biological activity of FSH and LH leading to a poor or suboptimal response to ovarian stimulation [5].
Genetic variations are thought to be sensitization to ovarian stimulation and poor or suboptimal response in some patients. Studies have demonstrated that several single-nucleotide polymorphisms (SNPs) on genes encoding follicle-stimulating hormone receptor (FSHR) or genes encoding the β subunit of LH hormone are involved in alteration response to ovarian stimulation. Therefore, some women in the suboptimal or suboptimal response group may carry one of these SNPs and require higher doses of gonadotropins or prolonged stimulation, which is different from the prediction of stimulus response based on the ovaries reserve marker. [6].
Hundreds of common variants or single-nucleotide polymorphisms (SNPs) on genes encoding FSHR have been identified. Of these, 4 gene variants (rs6165, rs6166, rs1394205, rs10835638) have been shown to be related to the decline in ovarian function and affect the effectiveness of controlled ovarian stimulation for women. have normal ovarian function [7].
- Polymorphism rs6165: Allele G at position 919 (c.919G>A). p.Ala307Thr. Amino acid substitution results in a change from a polar amino acid to a nonpolar, hydrophobic amino acid and the removal of a potential O-linked glycosylation site. Variant 307Ala/Ala (G/G) is associated with poor ovarian response. [8, 9]
• Polymorphism rs6166: Allele G at position 2039 (c.2039G>A). p.Ser680Asn. Amino acid substitution leads to a potential phosphorylation site in the intracellular domain of the receptor. The 680Ser/Ser (G/G) variant is associated with poor ovarian response [10, 11].
• Polymorphism rs1394205: The SNP is located in the promoter region of the FSHR gene, the upstream sequence of the translation initiation codon (c.-29G>A). Variant A/A is associated with poor ovarian response [12, 13].
• Polymorphism rs10835638: SNP located in the promoter region of the FSHB gene affects the transcription of the gene (c.-211G>T). T/T and G/T variants are associated with poor ovarian response [14].
Currently, GENTIS is performing FSH receptor gene polymorphism testing that allows the identification of genetic variants associated with response to ovarian stimulation. Detail:
FSHR: rs6165, rs6166, rs1394205
FSHB: rs10835638
Ovarian stimulation (KTBT) plays an important role in HTSS techniques. Antibiotic regimens can use FSH alone or in combination for high clinical pregnancy rates. There is no one ideal IVF regimen for all patients, so it is important to choose an appropriate IVF regimen based on many factors such as age of the wife, ovarian reserve, history of previous IVF attempts, and polymorphisms. FSH receptor gene. Appropriate use, proper use, careful monitoring of the FSH regimen will limit complications with a high clinical pregnancy rate.
References :
[1] Gallos, Ioannis D et al. “Controlled ovarian stimulation protocols for assisted reproduction: a network meta‐analysis.” The Cochrane Database of Systematic Reviews vol. 2017,3 CD012586. 9 Mar. 2017, doi:10.1002/14651858.CD012586
[2] Quaas, Alexander M, and Richard S Legro. “Pharmacology of medications used for ovarian stimulation.” _Best practice & research. Clinical endocrinology & metabolism_ vol. 33,1 (2019): 21-33. doi:10.1016/j.beem.2018.10.002
[3] Araújo, Valdevane R et al. “In vitro culture of bovine preantral follicles: a review.” Reproductive biology and endocrinology : RB&E vol. 12 78. 13 Aug. 2014, doi:10.1186/1477-7827-12-78
[4] Vaiarelli, A., Cimadomo, D., Ubaldi, N., Rienzi, L., & Ubaldi, F. M. (2018). What is new in the management of poor ovarian response in IVF?. Current opinion in obstetrics & gynecology, 30(3), 155–162. https://doi.org/10.1097/GCO.0000000000000452
[5] Bosch, E, et al. “Reduced FSH and LH Action: Implications for Medically Assisted Reproduction.” Human Reproduction, vol. 36, no. 6, 2021, pp. 1469–1480., https://doi.org/10.1093/humrep/deab065.
[6] Simoni, M et al. “Functional genetic polymorphisms and female reproductive disorders: Part I: Polycystic ovary syndrome and ovarian response.” Human reproduction update vol. 14,5 (2008): 459-84. doi:10.1093/humupd/dmn024
[7] Conforti, Alessandro et al. “Effect of Genetic Variants of Gonadotropins and Their Receptors on Ovarian Stimulation Outcomes: A Delphi Consensus.” Frontiers in endocrinology vol. 12 797365. 1 Feb. 2022, doi:10.3389/fendo.2021.797365
[8] Simoni, Manuela, and Livio Casarini. “Mechanisms in endocrinology: Genetics of FSH action: a 2014-and-beyond view.” European journal of endocrinology vol. 170,3 R91-107. 4 Feb. 2014, doi:10.1530/EJE-13-0624
[9] Alviggi, Carlo et al. “Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis.” Human reproduction update vol. 24,5 (2018): 599-614. doi:10.1093/humupd/dmy019
[10] Aittomäki, K et al. “Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure.” Cell vol. 82,6 (1995): 959-68. doi:10.1016/0092-8674(95)90275-9
[11] Alviggi, Carlo et al. “In Estimated Good Prognosis Patients Could Unexpected "Hyporesponse" to Controlled Ovarian Stimulation be Related to Genetic Polymorphisms of FSH Receptor?.” Reproductive sciences (Thousand Oaks, Calif.) vol. 23,8 (2016): 1103-8. doi:10.1177/1933719116630419
[12] Wunsch, Alain et al. “Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor.” Fertility and sterility vol. 84,2 (2005): 446-53. doi:10.1016/j.fertnstert.2005.02.031
[13] Lindgren, I et al. “Gonadotropin receptor variants are linked to cumulative live birth rate after in vitro fertilization.” Journal of assisted reproduction and genetics vol. 36,1 (2019): 29-38. doi:10.1007/s10815-018-1318-y
[14] Schüring, Andreas N et al. “Effects of the FSH-β-subunit promoter polymorphism -211G->T on the hypothalamic-pituitary-ovarian axis in normally cycling women indicate a gender-specific regulation of gonadotropin secretion.” The Journal of clinical endocrinology and metabolism vol. 98,1 (2013): E82-6. doi:10.1210/jc.2012-2780











