1. What is Preimplantation Genetic Testing (PGTest)?
According to the Ministry of Health, the infertility rate among Vietnamese families tends to rise increasingly, accounting for about 10-12% of couples of reproductive age. Therefore, IVF has become a crucial method to help infertile families conceive and have healthy children.
For a successful IVF treatment, PGTest - pre-implantation genetic analysis is one of the decisive factors with significant benefits. Approximately 80% of successful IVF cycles are related to embryo quality, making PGTest a “lifesaver” for cases of implantation failure and recurrent miscarriages.
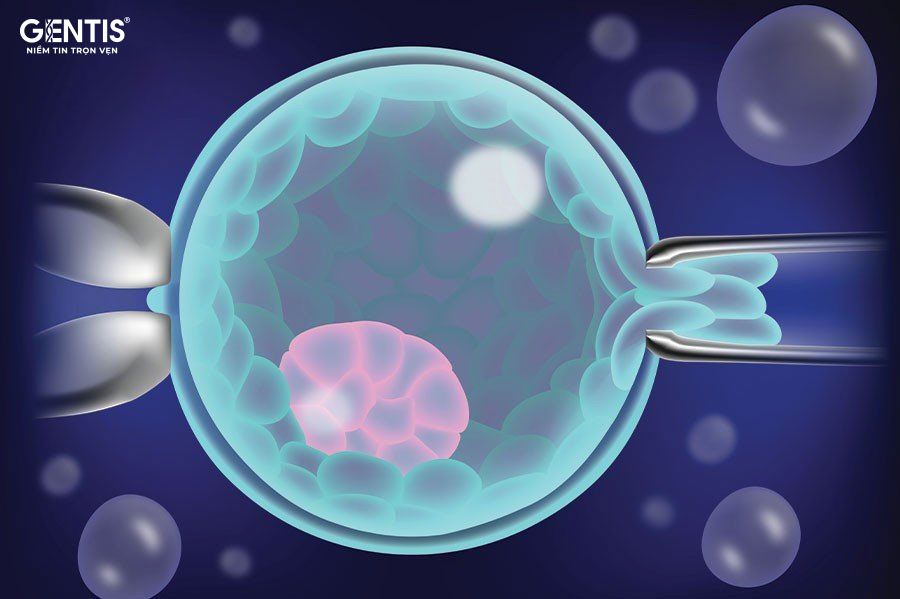
Pre-implantation genetic testing (PGT) is a test performed to analyze DNA from egg cells (polar bodies) or embryos (cleavage stage - morula or blastocyst stage) to identify HLA or genetic abnormalities. PGTest provides genetic health information about the embryos, helping fertility specialists select the best embryos for transfer, thus improving the chances of a successful pregnancy.
This technique increases the likelihood of conception , reduces miscarriage rates and multiple pregnancy risks, lowers the risk of single-gene disorders in the newborn, increases embryo survival rates, reduces the number of IVF cycles needed to achieve pregnancy, and saves time and costs for IVF treatments. As a result, PGTest is widely used in the field of reproductive assistance, particularly for couples undergoing IVF or intracytoplasmic sperm injection (IVF/ICSI).
2. Common Types of PGTest
Preimplantation genetic testing is divided into three main categories:
2.1. PGT- A (Preimplantation Genetic Screening for Aneuploidy)
PGT-A is a genetic test that detects chromosomal abnormalities in embryos before transferring them to the maternal uterus. It is indicated for:
- Advanced maternal age: 35 years old and above
- Multiple failed IVF attempts or recurrent miscarriages
- Family history of children born with chromosomal abnormalities
- Severe male infertility (AZF microdeletion)
2.2. PGT-SR (Preimplantation Genetic testing for structural chromosomal rearrangements)
PGT-SR is a genetic test that detects structural chromosomal abnormalities. It is indicated for couples where one partner carries structural chromosomal abnormalities such as balanced translocations, Robertsonian translocations, chromosomal deletions or duplications, or Angelman syndrome.
2.3. PGT-M (Preimplantation genetic testing for monogenic/single gene defects)
PGT-M is a preimplantation genetic testing for single-gene disorders. It is indicated for couples carrying gene mutations causing diseases like Thalassemia, spinal muscular atrophy, and cystic fibrosis, or for women carrying X-linked gene mutations (e.g., Hemophilia, Duchenne/Becker muscular dystrophy). Embryos screened by PGT-M without inherited disorders are prioritized for transfer to the maternal uterus to eliminate single-gene disorders in future generations.
3. PGTest Packages at GENTIS
Currently, GENTIS offers three PGTest kits: PGTest (PGT-A/SR), PGTest-M, and PGT Max 1 in order to help select embryos without chromosomal abnormalities and optimize embryo selection before transfer to the maternal uterus. GENTIS has also launched PGT Max 1 – a breakthrough test from PGTest with exclusive improvements unmatched by any other facility in Vietnam.

3.1. PGTest - A/SR
Analyzes DNA to detect aneuploidy in all 23 or 24 chromosome pairs and structural abnormalities (additions, deletions >5Mb)
- Method: Veriseq PGS – Illumina, USA. High resolution with over 3,000 data points; 100% sensitivity; 99.98% specificity; >1,000,000 total reads/sample, capable of detecting additions and deletions >5Mb
- Technology: Illumina Next-Generation Sequencing (NGS) – USA
- BlueFuse analytic software can identify over 130 syndromes related to chromosomal additions and deletions
- Sample: Embryo cells (day 3-5)
Note: Cannot detect balanced translocations
3.2. PGTest-M
Analyzes DNA to identify single-gene mutations in embryos related to diseases such as Thalassemia, Duchenne muscular dystrophy, hemophilia, RETT syndrome, etc.
- Method: SNP and STR analysis to detect inherited single-gene mutations from parents to embryos
- Technology: CE-Thermo, NGS Illumina – USA
- Sample: Embryo cells (day 3-5)
3.3. PGT Max-1
In addition to analyzing DNA for numerical and structural abnormalities across all 24 chromosomes and common syndromes caused by mutations >5Mb, PGT Max 1 can also detect two common microdeletions as small as 2Mb including 22q11.2 deletion (related to DiGeorge syndrome) and 1p36 deletion (related to 1p36 deletion syndrome).
- Method: Next-generation sequencing (NGS) (Illumina) and analysis of sequencing results using specialized bioinformatics software to identify aneuploidy in all 24 chromosomes and chromosomal additions/deletions
- Technology: Illumina NGS technology – USA and specialized analysis software
- Sample: Day 5 embryo biopsy
4. PGTest Procedure at GENTIS
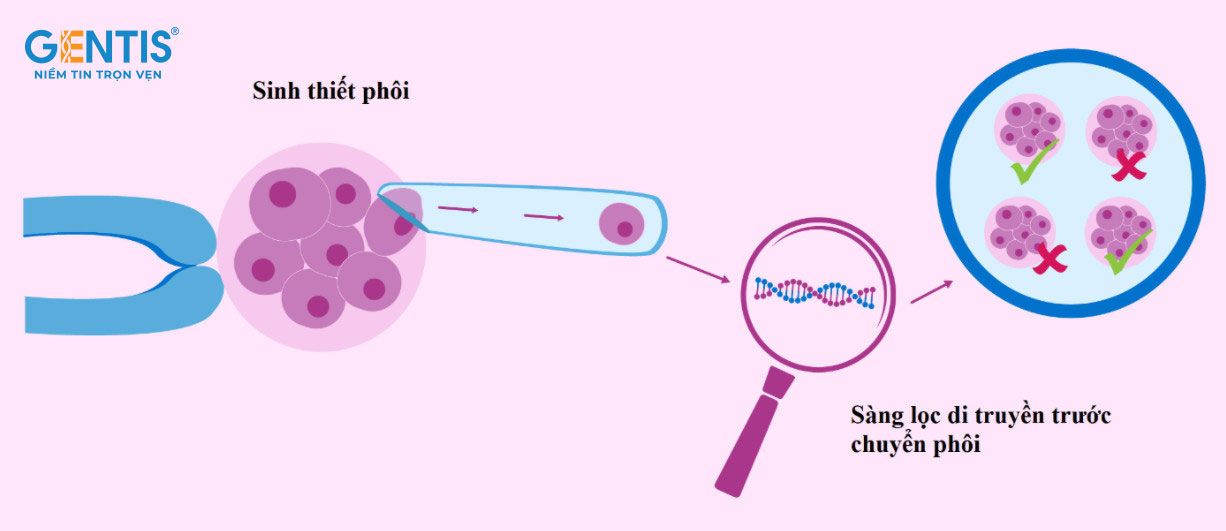
To ensure success and collaboration with IVF centers nationwide, PGTest at GENTIS follows a strict protocol to guarantee accuracy in the results. The process requires close coordination between the assisted reproduction unit and GENTIS International Laboratory from sample collection to preservation and transportation:
- Step 1: IVF is performed, and embryos are cultured in an embryo culture medium.
- Step 2: Embryo biopsy on day 3 or day 5, collecting a small number of cells – a crucial technique requiring precision and skill from the doctor.
- Step 3: Biopsied embryo cells are sent to GENTIS International Laboratory for analysis.
- Step 4: Embryos with normal results are selected for transfer. The remaining normal embryos are frozen for future use.
5. Why Choose PGTest at GENTIS?
Currently, PGTest can be performed at some specialized hospitals or assisted reproduction centers in Vietnam, but GENTIS is one of the few pioneering units capable of performing PGTest accurately and professionally using the most advanced technology and a team of top experts. GENTIS uses a complete next-generation sequencing system, chemicals, and analysis software from Illumina - USA. Globally, 80% of facilities use Illumina systems, with numerous scientific publications on accuracy, high resolution, and detection of small-scale disorders.
Moreover, Illumina's result turnaround time is much faster than other brands like PG-Seq, Chromint (China), etc. GENTIS's use of Illumina equipment provides a significant advantage over other units in supporting doctors and couples in having healthy children.
Furthermore, GENTIS continuously strives to improve service quality, applying the most advanced testing technologies and techniques to provide customers with comprehensive solutions. This contributes to enhancing human healthcare and protection. Through PGTest, GENTIS aims to bring happiness to infertile families across the country.











