PGT-M was first reported in 1990, indicating the genetic diagnosis of embryos at risk for an X-linked genetic disorder. With this technique we can examine the genome of embryos from a very early stage, from which abnormal fragments on a particular gene are inherited from the parents. From there, the embryos homozygous for the disease gene will be removed before being transferred to the mother's uterus. The effectiveness of this method has been proven to help limit the number of babies born with the disease as well as limit the need to terminate the pregnancy mid-term. [first]
PGT-M is used to help individuals or couples reduce the risk of having a child with a known genetic disorder caused by a single gene mutation such as cystic fibrosis, thalassemia, hemophilia Hemophilia, Duchenne muscular dystrophy, myelodysplasia, congenital muscular dystrophy, congenital adrenal hyperplasia,... [2]
In addition, in the future, this technique is also directed to be applied in the treatment of siblings of children who have been born but have the disease. With PGT-M, it is possible to diagnose human leukocyte antigen (HLA) matching of embryos with diseased siblings. Stem cells from umbilical cord blood of children born from embryos selected through PGT-M technique will help treat diseased siblings. [3]
Basically, to perform the PGT-M technique, couples need to have assisted reproduction by in vitro fertilization. The generated embryos will be raised to day 5. With the qualified day 5 embryos (blastocysts or blastocysts) biopsies will be performed (3-5 embryo cells are removed from the area where the cells will develop into the placenta. after that). Cell samples after biopsy will be applied special testing methods to detect disease-carrying genes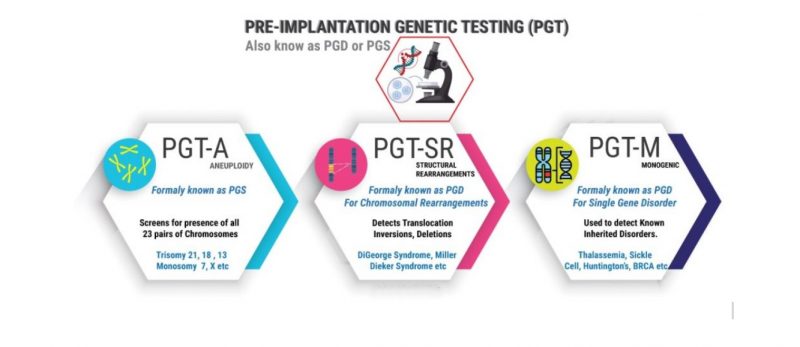
The technology used in the PGT-M test is SNP and STR analysis to detect single-gene mutations inherited from the father and mother to the embryo. Nucleotide polymorphism (SNP) microarrays detect single nucleotide pairs in genomic DNA that vary widely within a given species. An array typically evaluates about 300,000 SNPs spaced in the genome and provides one genotype per sample relative to the reference genome, thus identifying the entire chromosome aneuploidy, about 250 common structural aberrations. SNPs can also be used for molecular cytometry to detect the intensity ratio of B-to-A alleles at heterozygous loci, allowing for high-resolution deletion and duplication detection. Some limitations of SNP microarrays include limited ability to detect structural abnormalities below 5 MB, inability to detect balanced chromosomal rearrangements, and genetic abnormalities in a single pair. Linked spouses may not be detected because the SNP may be homozygous at each site. [4]
The PGT-M test is indicated in cases where there is a history of a spouse carrying the disease gene, or a family member with a genetic disease caused by a genetic mutation such as a recessive monogenic disease (eg, celiac disease). cysts), monogenic diseases (eg, Huntington's disease), sex-linked disorders (eg, Duchenne muscular dystrophy), chromosomal disorders (eg, translocations), predictor of cancer (breast cancer, cervical cancer ...) or determine HLA compatibility (to select embryos compatible with HLA antigens with a sick brother or sister requiring bone marrow transplant, blood transfusion. This person can donate bone marrow, cure your disease). For patients at risk of passing a genetic disease on to their children, PGT-M helps reduce risk, increase the likelihood of a healthy baby, and avoid ethically and emotionally challenging decisions. when deciding whether or not to terminate a pregnancy if a pregnancy has a genetic abnormality.
In clinical practice, the PGT-M test is often combined with performing the PGT-A/SR test before performing the PGT-M test to help comprehensively assess embryo quality and improve the success rate of assisted reproductive methods. Patients with monogenic disorders are mainly young patients and have a good prognosis, they still have a high risk of spontaneous abortion. In recent years, many studies have reported that when embryos are combined with PGT-A and PGT-M screening, pregnancy rates can be increased from 45% to 68% and spontaneous abortion rates decreased from 15 % down to 5%. [5]
Furthermore, the inclusion of PGT-A screening is appropriate for the majority of women undergoing IVF. All women are at risk of creating embryos with chromosomal abnormalities (trisomy 13, 18, 21, monosomy 23); As maternal age increases, the chance of embryos with chromosomal abnormalities increases. However, PGT-A can help women of all ages increase their chances of successful fertilization and embryo transfer. Chromosomal rearrangements (or chromosomal disorders) are mutations, deletions, duplications, inversions, and translocations of chromosome segments. People with chromosomal rearrangements are at risk of creating embryos with incorrect levels and sequences of genetic material, which often do not lead to successful conception. This phenomenon may be inherited or may occur spontaneously. Many people with balanced chromosomal rearrangements (segments, translocations) can remain healthy and unaware of their condition until they try to have children. For those with chromosomal rearrangements, PGT-SR may be performed to improve the chances of a healthy pregnancy. [6]
References:
[1] De Rycke, M., & Berckmoes, V. (2020). Preimplantation Genetic Testing for Monogenic Disorders. Genes, 11(8), 871. https://doi.org/10.3390/genes11080871
[2] Sullivan-Pyke, C., & Dokras, A. (2018). Preimplantation Genetic Screening and Preimplantation Genetic Diagnosis. Obstetrics and gynecology clinics of North America, 45(1), 113–125. https://doi.org/10.1016/j.ogc.2017.10.009
[3] Kakourou G., Kahraman S., Ekmekci G.C., Tac H.A., Kourlaba G., Kourkouni E., Sanz A.C., Martin J., Malmgren H., Giménez C., et al. The clinical utility of PGD with HLA matching: A collaborative multi-centre ESHRE study. Hum. Reprod. 2018;33:520–530. doi: 10.1093/humrep/dex384
[4] Sullivan-Pyke, C., & Dokras, A. (2018). Preimplantation Genetic Screening and Preimplantation Genetic Diagnosis. Obstetrics and Gynecology Clinics of North America, 45(1), 113–125. doi:10.1016/j.ogc.2017.10.009
[5] Unsal, E., Aktuna, S., Aydin, M., Ozer, L., & Baltacı, V. (2019). 69. IMPROVED IVF SUCCESS OF COMBINED PGT-M AND PGT-A APPLICATIONS. Reproductive BioMedicine Online, 39, e69-e70. https://doi.org/10.1016/j.rbmo.2019.04.122
[6] Sciorio, R., Tramontano, L., & Catt, J. (2020). Preimplantation genetic diagnosis (PGD) and genetic testing for aneuploidy (PGT-A): status and future challenges. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 36(1), 6–11. https://doi.org/10.1080/09513590.2019.1641194











