-
- 1800 2010
- EN

News
Array
(
[0] => stdClass Object
(
[id] => 1161
[id_crawler] =>
[category_product] => NULL
[thumbnail] => ht_spk_nha_trang_/edit_pgs_tam.jpg
[album] =>
[url_video] =>
[is_status] => 1
[is_featured] => 0
[is_form] => 0
[displayed_time] => 2023-11-29
[program] => 0
[number] => 1
[viewed] => 0
[type] =>
[type_career] =>
[level] =>
[address] =>
[address_career] =>
[expiration_time] => 0000-00-00
[created_time] => 2023-11-29 15:12:33
[updated_time] => 2024-08-28 14:14:24
[files] =>
[salary] =>
[time] =>
[created_by] => 63
[is_table_content] => 0
[language_code] => en
[slug] => GENTIS-co-sponsored-the-10th-open-central-region-and-central-highlands-obstetrics-and-gynecology-conference
[title] => GENTIS co-sponsored the 10th Open Central region and Central Highlands Obstetrics and Gynecology Conference
[description] => On November, 24th and 25th, the 10th Central region and Central Highlands Obstetrics and Gynecology Conference was successfully held in Nha Trang City, Khanh Hoa. GENTIS was honored to accompany the conference and introduced to delegates modern genetic tests.
[content] => The main theme of the symposium was "New challenges in obstetrics and gynecology and current practice perspectives". Through 4 seminar sessions, with 98 high-quality scientific reports, nearly 600 participants had the opportunity to update practical and in-depth knowledge on a number of topics such as: Prenatal pathology - Fetal medicine; New perspectives in Obstetrics and Gynecology, infertility; Advances in assisted reproductive techniques... Speakers are experienced leading experts in the field of obstetrics in Vietnam.
 The 10th Central region and Central Highlands Obstetrics and Gynecology Conference was held on April, 24th and 25th 2023
The 10th Central region and Central Highlands Obstetrics and Gynecology Conference was held on April, 24th and 25th 2023
In the second session of the conference, Nguyen Canh Chuong, MSc. MD. (Director of the Training and Steering Center of Hanoi Hospital of Obstetrics and Gynecology) presented the report: "Expanded NIPT: From a clinical perspective".
Dr. Chuong said that the expanded NIPT test will screen for more recessive genetic diseases with a combined frequency of 1/600 of acquired diseases, equivalent to the incidence of Down syndrome. Although the expanded NIPT has not been officially recommended by medical associations due to the lack of data evidence, according to Dr. Chuong, this may be a promising screening method.
In the report, Dr. Chuong also shared information about the units that are currently conducting expanded NIPT testing in Vietnam today. Accordingly, in addition to detecting common syndromes, GENTIS also offers extensive NIPT test kits, which can screen for 86 micro-deletion/duplication mutations and NIPT tests in cases of multiple pregnancies.
 Nguyen Canh Chuong, MSc. MD. (Director of Hanoi Hospital of Obstetrics and Gynecology) presented the report: "Expanded NIPT: From a clinical perspective"
Nguyen Canh Chuong, MSc. MD. (Director of Hanoi Hospital of Obstetrics and Gynecology) presented the report: "Expanded NIPT: From a clinical perspective"
In addition, GENTIS was also honored to provide information and data on FSH receptor polymorphic testing for the report of Assoc.Prof. Le Minh Tam PhD.MD. (Director of the Center for Reproductive Endocrinology and Infertility, Hue University of Medicine and Pharmacy Hospital) with the topic: "The role of genetics in low responsiveness of ovarian stimulation".
According to Associate Professor Tam, the identification and classification of patients in the group of poor or good ovarian response is the key to choosing the correct treatment strategy. Along with other factors affecting the ovarian stimulation response, Assoc. Prof. Tam believes that FSH receptor polymorphism is a useful tool for predicting and personalizing ovarian stimulation treatment, helping to achieve the right number of ovums for IVF in fewer stimulation cycles.
 Assoc. Prof. Le Minh Tam, PhD.MD. (Director of the Center for Reproductive Endocrinology and Infertility, Hue University of Medicine and Pharmacy Hospital) presented the report: "The role of genetics in low responsiveness of ovarian stimulation"
Assoc. Prof. Le Minh Tam, PhD.MD. (Director of the Center for Reproductive Endocrinology and Infertility, Hue University of Medicine and Pharmacy Hospital) presented the report: "The role of genetics in low responsiveness of ovarian stimulation"
GENTIS is one of the first units to implement the FSH receptor gene polymorphic test in Vietnam and is continuing to develop clinical studies in infertile women. FSH receptor gene polymorphism promises to be a useful test for doctors, adjuvanting the process of assisted reproductive treatment with good results.
In addition, the conference also had many other scientific reports such as:
- Challenges in current obstetrics and gynecology practice - Prof.Cao Ngoc Thanh PhD. (Vice Chairman of Vietnam Association of Obstetrics and Gynecology)
- Important updated recommendations in obstetrics and gynecology clinical practice - Le Quang Thanh, PhD. (Vice Chairman of the Vietnam Association of Obstetrics and Gynecology)
- Is it necessary to individualize ovarian stimulation in assisted reproduction? - Assoc. Prof. Nguyen Xuan Hoi, PhD.MD. (National Hospital of Obstetrics and Gynecology)
In addition to providing research data to the speakers, GENTIS was also honored to introduce to a large number of delegates participating in the conference the booth to display products with testing solutions from pre-pregnancy, during pregnancy and the newborn stage of the child. This test kit is a tool to support doctors to accompany pregnant mothers, and is the golden key to a perfect start for their children. In particular, GENTIS also sent doctors a lot of gratitude gifts.
 Product display booth of GENTIS attracted great attention from distinguished delegates
Product display booth of GENTIS attracted great attention from distinguished delegates
Through the 10th Open Conference on Obstetrics and Gynecology in the Central-Central Highlands, experts and doctors in the fields of obstetrics and gynecology and reproductive health had the opportunity to exchange experiences in scientific research, diagnosis and treatment. At the same time, they were updated with new clinical knowledge, and discussed and agreed on the development of technical expertise in obstetrics and gynecology. From there, it contributes to the development and improvement of the efficiency of medical health care for the community.
Once again, GENTIS would like to congratulate the Conference on its great success.
[content_more] =>
[meta_title] => GENTIS co-sponsored the 10th Open Central region and Central Highlands Obstetrics and Gynecology Con
[meta_description] => GENTIS was honored to accompany the conference and introduced to delegates modern genetic tests.
[meta_keyword] => GENTIS,Co-sponsored
[thumbnail_alt] =>
[post_id] => 1161
[category_id] => 4
)
[1] => stdClass Object
(
[id] => 1159
[id_crawler] =>
[category_product] => NULL
[thumbnail] => ht_spk_nha_trang_/bia_logo.jpg
[album] =>
[url_video] =>
[is_status] => 1
[is_featured] => 0
[is_form] => 0
[displayed_time] => 2023-11-20
[program] => 0
[number] => 1
[viewed] => 0
[type] =>
[type_career] =>
[level] =>
[address] =>
[address_career] =>
[expiration_time] => 0000-00-00
[created_time] => 2023-11-20 11:25:16
[updated_time] => 2024-08-28 14:08:45
[files] =>
[salary] =>
[time] =>
[created_by] => 63
[is_table_content] => 0
[language_code] => en
[slug] => GENTIS-Partners-with-the-10th-expanded-the-central-tay-nguyen-obstetrics-and-gynecology-conference
[title] => GENTIS Partners with The 10th Expanded The Central - Tay Nguyen Obstetrics and Gynecology Conference
[description] => The 10th Expanded The Central - Tay Nguyen Obstetrics and Gynecology Conference, organized by the Vietnam Obstetrics and Gynecology Association (VAGO), will be held on November 24-25, 2023 in Nha Trang, Khanh Hoa.
[content] => The 10th Expanded The Central - Tay Nguyen Obstetrics and Gynecology Conference, organized by the Vietnam Association of Gynecology and Obstetrics (VAGO), has attracted the attention of many experts, doctors, and researchers both domestically and internationally.

This is an opportunity for delegates working in the field of obstetrics and gynecology to enhance their knowledge and expertise through specialized presentations by domestic and international experts. At the same time, participating delegates will update general orientation information and current issues, engaging in discussions within the specialty.
Participating in this event, GENTIS Company is honored to bring many exciting programs, including:
- Exhibition booth of testing services at the conference
- Appreciation gifts for esteemed doctors
We sincerely invite all doctors to follow the conference agenda:




[content_more] =>
[meta_title] => GENTIS Partners with The 10th Expanded The Central - Tay Nguyen Obstetrics and Gynecology Conference
[meta_description] => The 10th Expanded The Central - Tay Nguyen Obstetrics and Gynecology Conference, organized by the Vietnam Obstetrics and Gynecology Association (VAGO), will be held on November 24-25, 2023 in Nha Trang, Khanh Hoa.
[meta_keyword] => GENTIS,partners
[thumbnail_alt] =>
[post_id] => 1159
[category_id] => 4
)
[2] => stdClass Object
(
[id] => 1158
[id_crawler] =>
[category_product] => NULL
[thumbnail] => huyen.8.2021/pgt1.png
[album] =>
[url_video] =>
[is_status] => 1
[is_featured] => 0
[is_form] => 0
[displayed_time] => 2023-11-20
[program] => 0
[number] => 1
[viewed] => 0
[type] =>
[type_career] =>
[level] =>
[address] =>
[address_career] =>
[expiration_time] => 0000-00-00
[created_time] => 2023-11-17 15:12:23
[updated_time] => 2024-08-28 14:04:53
[files] =>
[salary] =>
[time] =>
[created_by] => 62
[is_table_content] => 0
[language_code] => en
[slug] => Monogenic-diseases-that-can-be-detected-with-the-PGT-M--test-at-GENTIS
[title] => Monogenic diseases that can be detected with the PGT-M test at GENTIS
[description] => The PGT-M test is indicated for cases where the spouse carries the abnormal gene, or in the family there is a person with a genetic disease due to a genetic mutation that helps reduce the risk of giving birth to a child with a genetic disease from the parent, increasing the likelihood of having a healthy child.
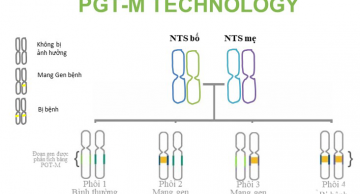
[content] => Meaning of the PGT-M test
PGT-M pre-embryo transfer genetic screening test helps couples choose gene-free/disease-free embryos to transfer into the mother's uterus to eliminate genetic mutations that cause disease in the next generation.

The PGT-M test will need to use the embryo sample and the reference sample as follows:
Sample of biopsied embryo (Day 3-5) or WGA product
Reference Sample
- Mandatory reference sample: Sample of Embryo's biological parents: 2-3ml of whole blood in an EDTA anticoagulant tube (with genetic test result information).
- Reference sample 3: Sample of the couple's existing child(s) who want to do PGT-M: 2-3ml of whole blood contained in an EDTA anticoagulant tube.
- Additional reference samples: in some cases, additional samples from the Embryo's grandparents may be requested: 2-3ml of whole blood contained in the EDTA anticoagulant tube.
Note: At a minimum, there must be a parent's reference sample.
The doctor will indicate embryo screening when it is known that the parent carries a mutation in one or more genes that cause the genetic disease. To determine whether you are a carrier of the abnormal gene or not, you can perform tests such as 13 hidden diseases combo, UltraGen at GENTIS to detect mutated genes.
PGT-M Test Procedure at GENTIS

Testing technology: MiSeq next-generation genome sequencing system.
+ Directly identify mutations on embryos (for point/indel mutations of less than 5 nucleotides).
+ Associated genetic analysis using SNPs located close to 2 sides of the mutation to be investigated.
+ Result turnaround time: Excluding public holidays
+ 12 days for 3 diseases: Myeloid atrophy, Duchenne muscle atrophy, Hemophilia, Thalassemia.
+ 30 days for diseases: G6PD enzyme deficiency, Phenylketonuria, Citrin deficiency, Wilson, Pompe, Cystic fibrosis, Fabry, Galactose metabolism disorder TYPE 1, Congenital hearing impairment, Adrenoleukodystrophy.
+ For other diseases, the result turnaround time depends on the types of gene mutations of the parents, for rare mutations, the time may be longer.
List of monogenic diseases performing PGT-M testing at GENTIS
|
No. |
DISEASE |
GENE NAME |
DISEASE INFORMATION |
|
1 |
Thalassemia |
HBA1, HBA2, HBB |
Thalassemia is a blood genetic disease that is associated with a gene mutation that regulates the production of hemoglobin. The main manifestations are anemia and hemochromatosis, and many patients with thalassemia (congenital hemolysis) need to receive blood transfusions and take iron chelators for life. |
|
2 |
Spinal muscular atrophy (SMA) |
SMN1 |
Spinal muscular atrophy (SMA) is a genetic disease that gradually destroys motor neurons – nerve cells in the brainstem and spinal cord that control essential musculoskeletal activity such as speaking, walking, breathing, and swallowing, leading to muscle weakness and atrophy. |
|
3 |
Duchenne muscular atrophy |
DMD |
Duchenne muscular atrophy is a disease that makes muscles weaker and less flexible over time. The muscles in the legs are usually one of the first and most visible affected sites. Children may be able to walk later, fall more easily, and have trouble climbing stairs or getting up. |
|
4 |
Hemophilia A |
F8 |
Hemophilia is a genetic bleeding disease associated with the sexual X chromosome caused by a decrease in factor VIII (hemophilia A) or factor IX (Hemophilia B) leading to thromboplastin dysplasia that delays blood clotting. People with Hemophilia, who are deficient in blood clotting factor, or have low levels of the factor compared to normal people, which makes it difficult for blood clots to form. |
|
5 |
Hemophilia B |
F9 |
|
|
6 |
Congenital adrenal hyperplasia (CAH) |
CYP21A2 |
Congenital adrenal hyperplasia (CAH) is one of the hereditary endocrine diseases, which occurs due to disorders of adrenal hormone synthesis. Causes acute adrenal insufficiency and affects the development of the genital organs. |
|
7 |
G6PD deficiency |
G6PD |
As the most common enzymatic disease on X chromosomes, boys are more susceptible to it than girls. Red blood cells will be destroyed, causing anemia due to hemolysis, mental and motor retardation, jaundice, and dark urine. |
|
8 |
Phenylketonuria |
PAH |
The disease is characterized by intolerance to the amino acid Phenylalanine in daily meals. If not diagnosed early after birth and treated promptly, the disease can cause irreversible brain damage and mental retardation. |
|
9 |
Citrine deficiency |
SLC25A13 |
The disease causes jaundice and cholestasis in infants. Children under 1 year old have a history of low weight and growth restrictions. Beyond 1 year old, children have symptoms such as hypoglycemia, pancreatitis, severe fatigue, loss of appetite, and decreased quality of life. Heavy weight usually begins suddenly in adulthood. Neurological symptoms appear. |
|
10 |
Wilson's disease |
ATP7B |
It is a type of genetic disorder due to genetic abnormalities that causes the body to not eliminate residual copper, leading to the accumulation of copper in body tissues (liver, brain, eyes and other organs) and causing toxicity to the patient, even life-threatening |
|
11 |
Pompe’s disease |
GAA |
Pompe's disease (PD) is a genetic metabolic disorder caused by a deficiency of acid α-glucosidase (GAA), which leads to the accumulation of glycogen in lysosomes, mainly in the skeletal muscle and heart muscle as well as the nervous system. Symptoms that appear immediately after the child is born include: weak muscle tone, eating difficulty, slow weight gain, heart defects, shortness of breath, swelling of the tongue, enlarged liver, weakened liver function, hearing loss,... |
|
12 |
Fabry's disease |
GLA |
Fabry's disease is a type of inherited metabolic disorder, characterized by a deficiency of alpha-Galactosidase enzyme that causes a number of pathological conditions such as keratotic angiomas, corneal opacity, limb paresthesia, renal failure, heart failure, etc. |
|
13 |
Metabolic disorder of galactose type 1 |
GALT |
This is a disease that causes disorders in the conversion of galactose into glucose, causing children to not be able to convert this sugar into energy to be used but accumulate in the blood. Early manifestations in the first few weeks after birth such as poor feeding or skipping breastfeeding, diarrhea, vomiting, coma, physical examination showing jaundice, subcutaneous hemorrhage, hepatomegaly, cataracts. |
|
14 |
Congenital hearing loss |
GJB2 |
60% of children are born with bilateral birth defects due to genetics And abnormalities of the GJB2 gene (recessive gene on autosomal chromosomes) are the most common cause of congenital hearing loss in children. Screening is no later than 1 month old for early detection and intervention, limiting the impact on children's language development. |
|
15 |
Metachromatic leukodystrophy (MLD) |
ARSA |
Metachromatic leukodystrophy (MLD) is one of the diseases related to lipid storage, which causes a toxic accumulation of abnormal fats, which interfere with normal fats and proteins in the myelin shell. From 1-2 years of age, decreased muscle tone, behavioral decline, later paralysis, visual atrophy. Precocious puberty; from 5 to 10 years old, paralysis, epilepsy, death after 10-20 years. |
|
16 |
Adrenal leukodystrophy (ALD) |
ABCD1 |
Adrenal leukodystrophy is a genetic disorder that occurs mainly in men, affecting the nervous system and adrenal glands. This is the most common hereditary disorder (peroxisome), caused by a gene mutation on chromosome Xq28, which has now isolated more than 200 mutations. |
|
17 |
Ornithine transcarbamylase deficiency (OTC) |
OTC |
Ornithine transcarbamylase (OTC) deficiency is a genetic disorder that causes ammonia to build up in the blood. Infants with OTC deficiency may lack energy (apathy) or do not want to eat, and have poorly controlled breathing rates or body temperature. Complications due to ornithine transcarbamylase deficiency may include developmental delays and intellectual disability. Progressive liver damage can also occur. |
|
18 |
Congenital hypothyroidism |
SLC26A4 |
It is a thyroid hormone deficiency disease caused by thyroid development problems or thyroid hormone biosynthesis disorders. |
|
19 |
Smith-Lemli-Opitz syndrome |
DHCR7 |
Smith-Lemli-Opitz syndrome is a disease that disrupts the biosynthesis of cholesterol (reduced). Manifestations of intellectual retardation (100%); microcephaly (90%), cleft palate, small palate, heart defects; underdeveloped external genital organs in men; musculoskeletal deformities. |
|
20 |
HLA conformity |
Determine whether the embryo is suitable for HLA for the sick person so that after the baby is born, there will be a plan to store umbilical cord blood for stem cell transplantation. |
|
|
21 |
Cystic fibrosis (CF) |
CFTR |
Cystic fibroids, also known as cystic fibroids, are genetic disorders that cause the body to produce excessive sweat and mucus. The disease affects the functioning of the lungs, digestive system and genital organs. |
|
22 |
Cartilaginous dysplasia (Achondroplasia) |
FGFR3 |
Achondroplasia is a rare group of inherited bone disorders. This is the most common form that has ever been called dwarfism, in which a child's arms and legs are short compared to their body length. The average height of adult males and females with achondroplasia is about 132cm and about 124cm, respectively. |
|
23 |
Vitreous bone disease |
COL1A1, COL1A2 |
Vitreous bone is a phenomenon of brittle, fragile bones, and incomplete bone formation. The main reason for this condition is a disruption in the genetic process that makes bones fragile even under the influence of a small impact or injury. |
|
24 |
Autosomal dominant polycystic kidney disease (ADPKD) |
PKD1, PKD2 |
Hereditary polycystic kidney disease is a genetic disorder in which clusters of cysts develop inside the kidneys, causing the kidneys to gradually increase in size and decline in function over time. |
|
25 |
Familial hypercholesterolemia (FH) |
LDLR |
People with familial hypercholesterolemia are basically born with high levels of LDL cholesterol. People's cholesterol levels tend to increase with age, however, people with familial hypercholesterolemia have very high levels of baseline LDL cholesterol and rise faster over time. |
|
26 |
Alport Syndrome |
COL4A5 |
Alport syndrome (hereditary nephritis) is a disease that damages the small blood vessels in the kidneys by attacking the renal platelets (the smallest filtration unit in the kidneys), leading to kidney disease and eventually kidney failure. |
|
27 |
Brugada syndrome |
SCN5A |
Brugada syndrome is one of the most common causes of sudden death with causes related to cardiovascular diseases. The disease has the potential to cause life-threatening arrhythmias. People with this syndrome are at high risk of experiencing arrhythmias that originate from the ventricles located in the lower part of the heart. |
|
28 |
Ichthyosis vulgaris |
FLG |
Ichthyosis is a condition in which the skin is damaged mainly due to genetics. Dead skin cells accumulate into patches of skin, thick and dry pieces like fish scales on the surface of the skin. The disease usually appears between the ages of 0 and 7 years, and there are even cases where the disease appears immediately after childbirth. |
|
29 |
Marfan’s syndrome |
FBN1 |
Marfan’s syndrome in people with long limbs and long fingers is a genetic disorder that affects connective tissue. The most obvious signs of this disorder include height, thinness, abnormally long limbs, disproportionately long fingers and toes, protruding or concave sternum, curved spine, loose joints, large and flat feet, severe myopia. |
|
30 |
Hemophagocytic Lymphohistiocytosis (HLH) |
PRF1 |
Hemophagocytic syndrome is a rare blood disease with a mortality rate of 15-60%, a common disease in children under 10 years old, for the primary form usually appears in children under 1 year old while the secondary form does not develop until about 6 years old. This is a syndrome in which histiocytic hyperactivity increases leading to erythrocytes, platelets, and white blood cells to be phagocytized. |
PGT - The Golden Key to Finding Children for Families
The invention of in vitro fertilization (IVF) has helped millions of infertile couples realize their dream of having children after years of waiting. However, couples needing IVF are often older or have issues that reduce the quality of their eggs/sperm. Therefore, embryo transfer often fails or, if successful, may carry risks of having children with birth defects.

Preimplantation genetic testing (PGT) has become an important solution to help couples undergoing IVF achieve successful embryo transfer and give birth to healthy babies.
PGT has become the "golden key" in families' journey to find children, helping to:
- Increase pregnancy rates per embryo transfer
- Reduce miscarriage rates
- Reduce the risk of multiple pregnancies
- Increase the chance of having healthy children
- Reduce the time and resources needed
Given the immense significance of PGT testing, couples should consider getting tested if recommended by their doctor to increase their chances of successful pregnancy and giving birth to a healthy baby.
History of PGT Testing Development at GENTIS
GENTIS Testing Center is proud to be a pioneer in PGT testing in Vietnam, helping countless families turn their dream of having children into reality. Let's take a look at GENTIS's impressive milestones in its 10 years of PGT testing development.
.jpg)
2013: The first NGS Illumina system for PGS (now PGT) testing was imported to GENTIS.
2014: PGT testing was assessed for its value in use at some of the first IVF centers in Vietnam.
2015:PGT testing was officially implemented in Vietnam.
2016-2018: PGT testing volume increased significantly, with more and more IVF centers referring patients for PGT testing.
2019-2022: PGT became a familiar test for IVF centers nationwide.
2023: GENTIS became the first organization in Vietnam to achieve ISO 15189:2012 certification for PGT-A testing services, a testament to the tireless efforts of all GENTIS Testing Center staff.

Up to now, GENTIS has performed testing for approximately 30 IVF centers in Vietnam, with a volume of over 30,000 embryos per year.
In particular, GENTIS has published numerous scientific research papers on PGT in reputable international journals and presented reports at international conferences, including:
- The research paper "Development and clinical application of a preimplantation genetic testing for monogenic disease (PGT-M) for beta thalassemia in Vietnam" was published in the Journal of Assisted Reproduction and Genetics by the American Society for Reproductive Medicine (ASRM).
- A report on the topic "PGT-M for rare diseases in Vietnam" at the ASPIRE 2021 conference.
- A report on "One case of preimplantation genetic testing for Marfan’s syndrome in Vietnam" at the 19th International Preimplantation Genetic Diagnosis Society (PGDIS) conference.
- A report on "A comprehensive pre-implantation genetic test for alpha and beta thalassemia disease" at the 19th International Preimplantation Genetic Diagnosis Society (PGDIS) conference.
With a 10-year journey of implementing and developing PGT testing, GENTIS has made a significant contribution to helping couples select genetically high-quality embryos, increasing the chances of successful pregnancy when undergoing IVF, ensuring that children born are healthy and free from the screened genetic syndromes.
[content_more] => [meta_title] => GENTIS's History of PGT Testing Development [meta_description] => GENTIS is a pioneer in PGT testing in Vietnam. Since 2013, GENTIS has established infrastructure and medical equipment to perform PGT tests. [meta_keyword] => Gentis,PGTTESTING,PGT [thumbnail_alt] => [post_id] => 1157 [category_id] => 4 ) [4] => stdClass Object ( [id] => 1156 [id_crawler] => [category_product] => NULL [thumbnail] => z4863539179751_fe549b61454a17ee737252ed5710a7f6.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 1 [displayed_time] => 2023-11-16 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2023-11-16 09:36:01 [updated_time] => 2024-08-28 13:55:29 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => GENTIS-launches-new-sperm-DNA-Test-to-aid-in-male-infertility-dianosis [title] => GENTIS Launches New Sperm DNA Test to Aid in Male Infertility Diagnosis [description] => GENTIS has officially launched the Sperm DNA test, which quantifies the percentage of fragmented sperm DNA based on dye color changes using flow cytometry (analyzing up to 10,000 sperm cells). This test helps explain recurrent miscarriages, unexplained infertility, and failed IVF attempts in couples. Notably, this test is cost-effective, making it accessible to all men. [content] =>Today, men are increasingly concerned about their reproductive health. Recognizing this, GENTIS has researched and launched the Sperm DNA test to provide a comprehensive assessment of sperm quality. This test is crucial, enabling doctors to predict a man's reproductive potential and recommend the most appropriate intervention (choosing assisted reproductive methods).

1. Why should I get a Sperm DNA test?
Sperm DNA damage can be defined as any chemical alteration from the normal structure of DNA. Among these changes, sperm DNA fragmentation (SDF) is one of the most common disorders affecting genetic material in the form of single or double-strand breaks

There are various types of sperm DNA damage, including:
- Base mispairing (Caused by replication errors)
- Base loss (no base at the position)
- Base alteration (oxidation, alkylation, deamination)
- Cross-linking and adducts (Caused by carcinogens and ROS
- Single-strand breaks (SSB) and double-strand breaks (DSB) (Caused by ROS, toxic chemicals, ionizing radiation, broken replication forks)
Any changes in sperm DNA can lead to sperm DNA fragmentation (SDF) and affect the chances of natural conception or the outcome of assisted reproductive technology (ART).
Sperm DNA fragmentation is characterized by both single-strand breaks (SSB) and double-strand breaks (DSBs) of DNA, and is particularly common in the semen of men with impaired fertility. High levels of sperm DNA fragmentation are one of the causes of male infertility, affecting embryo development, implantation, and pregnancy in both natural and assisted reproduction.
Therefore, Sperm DNA served as an important supplementary test in diagnosing male infertility. This test helps doctors better screen cases of unexplained infertility, thereby guiding appropriate interventions to shorten treatment time, cost, and increase the chances of having children for couples.
2. Who should get a Sperm DNA test?

According to GENTIS experts, men should get a Sperm DNA test if they fall under the following circumstances:
* Unexplained male infertility or unclear causes.
* Recurrent miscarriages.
* Clinical symptoms of varicocele.
* Lifestyle-related risk factors.
* Before or after the failure of assisted reproductive techniques (ART) such as IUI, IVF, ICSI.
* Recurrent miscarriages after ICSI.
3. What makes the Sperm DNA test at GENTIS special?
- Advanced Technology: Analysis on the Flow cytometry system (analyzing up to 10,000 sperm cells). The advantage of the Flow cytometry system is its stability, accuracy, and high performance in Sperm DNA testing.
- Automated and Fast Process
- Result Delivery Time: 24 hours
- Cost: The most reasonable, making Sperm DNA testing accessible to all men.

With ISO-certified standards, work processes, and a team of leading experts in genetics and biotechnology, GENTIS is committed to providing customers with high-quality, affordable Sperm DNA testing and the most professional service. Notably, the Sperm DNA test provides strong support in diagnosing genetic factors related to male infertility, thereby helping doctors develop appropriate treatment regimens to overcome male infertility.
The workshop was attended by leading experts, including Ho Manh Tuong MD., MSc, Head of the Assisted Reproduction Unit at My Duc Hospital and Secretary-General of Ho Chi Minh City Society for Reproductive Medicine, Prof. Nguyen Dinh Tao, PhD, President of the Assisted Reproduction Society, etc. And more than 200 delegates, including leading professors, obstetricians, embryologists, and leaders of assisted reproduction centers from leading hospitals, medical facilities, and testing centers nationwide, also participated.
The colloquium on Preimplantation Genetic Testing is a prestigious scientific event highly regarded by the medical community in the field of assisted reproduction. It is a reliable forum for experts, doctors, embryologists, and healthcare professionals to exchange, share, and learn from each other.
The workshop was divided into four sessions, with a total of 22 research papers presented in the plenary session and one poster presentation by leading experts nationwide. The presenters focused on discussing preimplantation genetic testing (PGT).
Opening Session I, Doan Thi Hang, MD, PhD, Deputy Director of Military Institution of Clinical Embryology and Histology, presented a paper on "The history of PGT development in Vietnam." In her presentation, Dr. Hang clearly outlined the history of PGT development worldwide and in Vietnam. The presentation pointed out that GENTIS was the first unit to introduce PGT to Vietnam by importing the first Illumina NGS system in 2013. By 2015, PGT was officially applied in Vietnam.
In 2023, GENTIS became the first unit in Vietnam to achieve ISO 15189:2012 for PGT-A testing services. With the development and advancement of science and technology, strategies for analyzing the genetic material of embryos are constantly being improved, thereby enhancing the quality of testing and the effectiveness of PGT in Assisted Reproduction.
 Dr. Doan Thi Hang, Deputy Director of Military Institution of Clinical Embryology and Histology, presented a report on "The history of PGT development in Vietnam."
Dr. Doan Thi Hang, Deputy Director of Military Institution of Clinical Embryology and Histology, presented a report on "The history of PGT development in Vietnam."
To ensure the quality of PGT testing, technical factors and technology are of paramount importance. This information was presented by Nguyen Quang Vinh, MSc, Director of the GENTIS Testing Center, in his report titled "Technical factors and technology in controlling and improving the quality of PGT testing."
Mr. Vinh stated that GENTIS is currently using Next-Generation Sequencing (NGS) technology from Illumina (USA) for PGT testing. The outstanding advantage is that it has been proven to have 100% accuracy compared to the aCGH method and detects mosaicism more effectively than aCGH (from 20% to 80%). Additionally, it offers higher resolution in detecting chromosomal structures compared to aCGH (detecting chromosomal deletions of up to 5 Mb; currently, it has been optimized to detect two more microdeletions of 2 Mb in size: 22q11 and 1p36).
 Nguyen Quang Vinh, MSc, Director of the Testing Center, presented a report on "Technical factors and technology in controlling and improving the quality of PGT testing".
Nguyen Quang Vinh, MSc, Director of the Testing Center, presented a report on "Technical factors and technology in controlling and improving the quality of PGT testing".
Additionally, the PGT laboratory must adhere to the ESHRE guidelines for PGT laboratory requirements, with a particular focus on sample transportation from the IVF center. Furthermore, the PGT laboratory must participate in the annual external quality assessment program of GENQA and obtain ISO 15189:2012 certification. All of these requirements are fully complied with and implemented by GENTIS to control and enhance the quality of PGT testing
 Assoc. Prof. Ho Sy Hung, PhD from Hanoi Medical University presented a report on "The application of PGT testing in the detection and screening of diseases in preimplantation embryos in Vietnam".
Assoc. Prof. Ho Sy Hung, PhD from Hanoi Medical University presented a report on "The application of PGT testing in the detection and screening of diseases in preimplantation embryos in Vietnam".
According to experts, PGT testing can be applied to detect and screen diseases in preimplantation embryos. This has been clearly presented by Assoc. Prof. Ho Sy Hung, PhD in his report. Dr. Hung stated that in Vietnam, PGT tests for screening genetic diseases such as PGT-M and PGT Max 1 are being increasingly optimized in terms of time and cost, giving more families with genetic diseases the opportunity to access this technology.
 Specialist level 1 doctor Nguyen Van Thong from Hung Vuong Hospital presented a paper on "Genetic counseling for transferring mosaic embryos."
Specialist level 1 doctor Nguyen Van Thong from Hung Vuong Hospital presented a paper on "Genetic counseling for transferring mosaic embryos."
The topic of counseling for transferring mosaic embryos, presented by Specialist level 1 doctor Thong, also attracted significant attention from the delegates. Dr. Thong suggested that counseling for mosaic embryos should prioritize factors such as the percentage of mosaicism in the biopsy sample, the number of chromosomes involved: 1 or more chromosomes, the type of chromosomes, complete or partial chromosome mosaicism, Monosomy or Trisomy type. Additionally, healthcare providers should provide multidisciplinary counseling and follow-up for embryos with mosaicism.
Furthermore, the conference featured numerous other scientific presentations, including
- “Choosing ICSI or IVF fertilization methods for PGT cases" by Nguyen Thi Cam Van, MD, MSc from Vinmec International General Hospital.
- "The efficacy of preimplantation genetic testing for aneuploidy (PGT-A) in patients with poor ovarian response” - M.S. Ho Thi My Trang at My Duc Phu Nhuan Hospital.
- “The future of preimplantation genetic screening for embryos at risk of gynecological cancer gene mutations: Potential and challenges.” - Nguyen Hai Phuong, MD, MSc at National hospital of Obstetrics and Gynecology.
These presentations provided healthcare professionals with updated knowledge about PGT and offered new perspectives on this testing method, enabling them to better support infertile couples.
As a pioneer in introducing PGT to Vietnam and a global partner of major companies like Illumina (USA), Qiagen, Sigma, etc, GENTIS has played a significant role in helping couples select genetically healthy embryos, increasing the success rates of IVF and ensuring the birth of healthy babies, not affected by any genetic syndromes detected through screening
GENTIS's PGT tests have become widely adopted by IVF centers nationwide, including:
- PGT-A/SR: DNA analysis to detect abnormalities in the number (aneuploidy) of all 24 pairs of chromosomes and structural abnormalities of chromosomes in embryos.
- PGTest-M: diagnose single-gene genetic disorders.
- PGT-Max 1: an advanced genetic test that uses groundbreaking technology to detect smaller structural abnormalities in chromosomes, specifically microdeletion/microduplication greater than 2 Mb in size, associated with a number of common genetic diseases and syndromes.
- ASEM test: a genetic analysis of the embryo culture environment.
Through this colloquium on Preimplantation Genetic Testing, GENTIS aims to strengthen its partnership with leading medical institutions across the country, providing a comprehensive testing ecosystem to better serve the healthcare needs of Vietnamese people. By doing so, GENTIS is realizing its vision of bringing happiness to infertile families.
[content_more] => [meta_title] => GENTIS Sponsored and Presented at the Colloquium on Preimplantation Genetic Testing [meta_description] => GENTIS was honored to be the main sponsor and to participate in presenting at the event, contributing to its overall success [meta_keyword] => Gentis,mainsponsor,presented,event [thumbnail_alt] => [post_id] => 1155 [category_id] => 4 ) [6] => stdClass Object ( [id] => 1152 [id_crawler] => [category_product] => NULL [thumbnail] => hội-thảo-pgt-ngang.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 1 [displayed_time] => 2023-11-07 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2023-11-07 14:50:27 [updated_time] => 2024-08-28 13:31:37 [files] => [salary] => [time] => [created_by] => 63 [is_table_content] => 1 [language_code] => en [slug] => GENTIS-Proudly-Sponsors-Pre-implantation-Genetic-Testing-Symposium [title] => GENTIS Proudly Sponsors Pre-implantation Genetic Testing Symposium [description] => The Pre-implantation Genetic Testing Symposium will be held at 8 AM on Saturday, November 11, 2023, at Caravelle Saigon Hotel. [content] =>Organized by Hosrem, the symposium has attracted the attention of numerous experts, doctors, researchers, and embryologists. This event provides an opportunity for professionals in the field of assisted reproduction to exchange knowledge and enhance their expertise.

GENTIS is honored to be the main sponsor of the Pre-implantation Genetic Testing Symposium. As part of our participation, we will be hosting an informative booth showcasing our advanced PGT testing services. Additionally, we will be conducting engaging mini-games with exciting prizes for all attending physicians.
Our esteemed colleague, Mr. Nguyen Quang Vinh, Head of Medical Laboratory Center, will be delivering a keynote speech entitled "Technical and Technological Factors in Quality Control and Improvement of PGT Testing".
We cordially invite all physicians to join us!
[content_more] => [meta_title] => GENTIS Proudly Sponsors Pre-implantation Genetic Testing Symposium [meta_description] => The Pre-implantation Genetic Testing Symposium will be held at 8 AM on Saturday, November 11, 2023, at Caravelle Saigon Hotel. [meta_keyword] => GeneticTesting,Gentis,Sponsors,Symposium [thumbnail_alt] => [post_id] => 1152 [category_id] => 4 ) [7] => stdClass Object ( [id] => 1151 [id_crawler] => [category_product] => NULL [thumbnail] => ht-sk_2023/thue_thủ_đo_2023/286936ed7411a34ffa00.jpg [album] => [url_video] => [is_status] => 1 [is_featured] => 0 [is_form] => 0 [displayed_time] => 2023-10-27 [program] => 0 [number] => 1 [viewed] => 0 [type] => [type_career] => [level] => [address] => [address_career] => [expiration_time] => 0000-00-00 [created_time] => 2023-10-27 16:42:14 [updated_time] => 2024-08-28 13:27:23 [files] => [salary] => [time] => [created_by] => 62 [is_table_content] => 0 [language_code] => en [slug] => GENTIS-was-recognized-as-an-exemplary-taxpaying-business-in-the-Capital-City-for-2022 [title] => GENTIS was recognized as an exemplary taxpaying business in the Capital City for 2022. [description] => On the morning of October 26th, the Hanoi Tax Department convened the "Commendation of Exemplary Taxpayers in the Capital City for 2022" conference. GENTIS was honored as one of the outstanding taxpaying enterprises that made substantial contributions to the national and social budget over the year of 2022. [content] =>
The "Commendation of Exemplary Taxpayers in the Capital City" conference aimed to recognize the contributions of enterprises and entrepreneurs. It also served to encourage and inspire the business community to continue overcoming challenges and developing their production and business operations. The conference was attended by Mr. Ha Minh Hai - Hanoi Deputy Mayor, Mr. Vu Manh Cuong - Director of Hanoi Tax Department, Mr. Mai Son - Deputy General Director of General Department of Taxation Mai Son, representatives from government agencies, media outlets, and participating enterprises.

At the event, GENTIS was honored to receive a Certificate of Merit from the Hanoi Deputy Mayor. Since its establishment, GENTIS has consistently complied with and implemented tax policies and laws, fulfilling its tax obligations to the national budget. GENTIS not only contributes a significant amount to the national budget, but also plays a leading and critical role in ensuring income and social security for its employees, thereby contributing to the socioeconomic development of the capital city in particular and the country in general.

Mr. Do Manh Ha, General Director of GENTIS, expressed his gratitude and pride at the commendation ceremony: "Contributing to the community is the responsibility of every enterprise. GENTIS is honored to be recognized by the Hanoi Tax Department for our positive contributions to the country. We commit to continuing to contribute to the economic development and social welfare of the capital city in particular and the country in general. GENTIS will continue to maintain the highest standards in all of our activities, including strict compliance with tax policies and laws, as well as transparency in our business operations."
Tax payment is not only an obligation, but also a crucial stepping stone that helps the country build a strong financial foundation to advance its development and take a leading position in the Southeast Asian region. Above all, GENTIS aspires to become a brand that is endorsed by the country, affirming its mission and stature on the journey to enhance the physical and intellectual well-being of the Vietnamese people.
[content_more] => [meta_title] => GENTIS was recognized as an exemplary taxpaying business in the Capital City for 2022. [meta_description] => On the morning of October 26th, the Hanoi Tax Department convened the "Commendation of Exemplary Taxpayers in the Capital City for 2022" [meta_keyword] => Gentis,Recognized,Business [thumbnail_alt] => [post_id] => 1151 [category_id] => 4 ) )GENTIS co-sponsored the 10th Open Central region and Central Highlands Obstetrics and Gynecology Conference
GENTIS Partners with The 10th Expanded The Central - Tay Nguyen Obstetrics and Gynecology Conference
Monogenic diseases that can be detected with the PGT-M test at GENTIS
GENTIS's History of PGT Testing Development
GENTIS Launches New Sperm DNA Test to Aid in Male Infertility Diagnosis
GENTIS Sponsored and Presented at the Colloquium on Preimplantation Genetic Testing
GENTIS Proudly Sponsors Pre-implantation Genetic Testing Symposium
GENTIS was recognized as an exemplary taxpaying business in the Capital City for 2022.
Please fill in the information below to receive our supports and consultations!
Please fill in the information below to receive our supports and consultations!